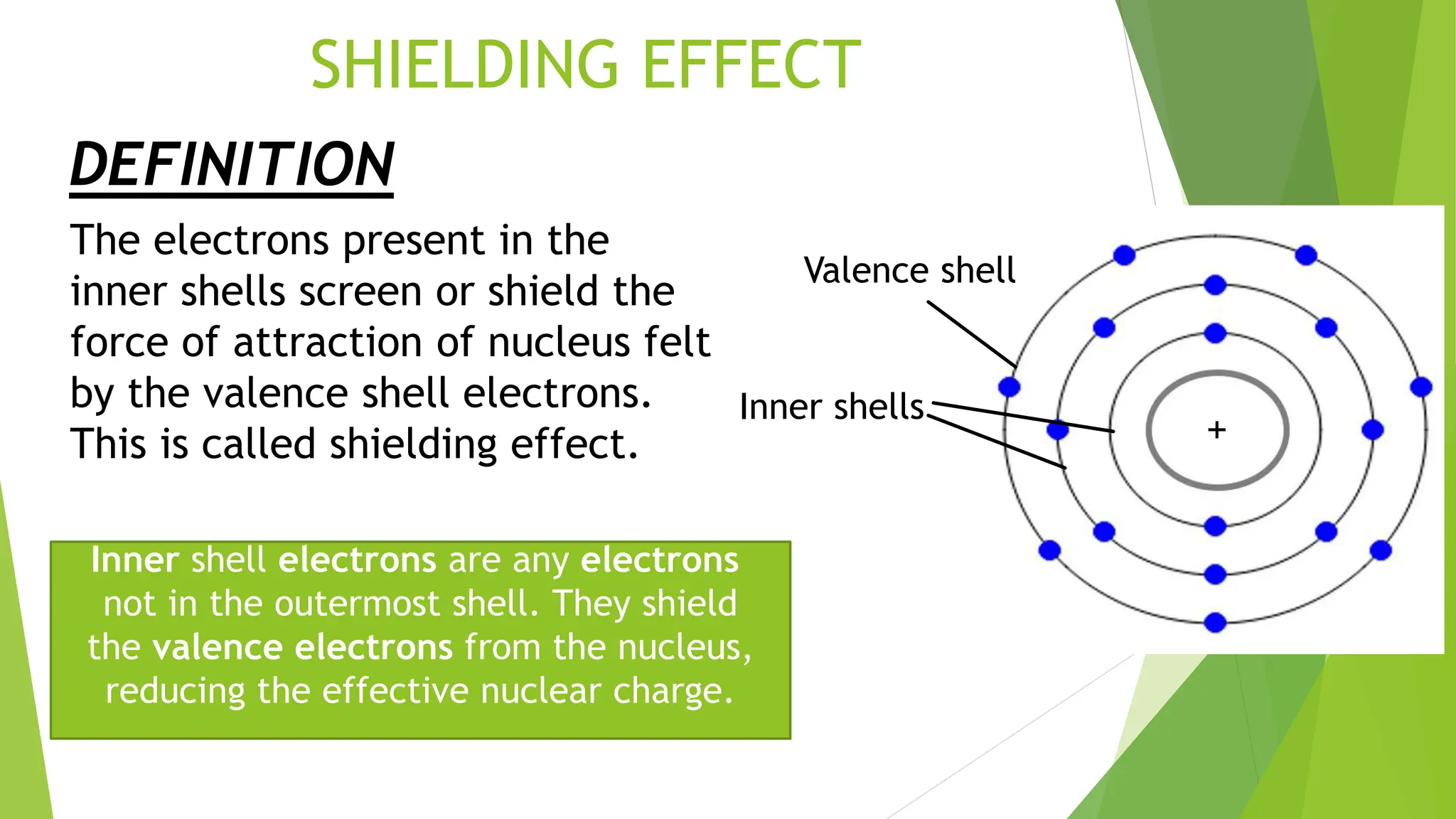
What Is Shielding Effect In Periodic Table . Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. Shielding is caused by the combination of. Hence, the nucleus has less grip on the. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. This lecture is about shielding effect and screening effect in the periodic table.
from www.slideshare.net
This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This lecture is about shielding effect and screening effect in the periodic table. Shielding is caused by the combination of. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons.
Shielding Effect periotic table and preodicity of property PPT
What Is Shielding Effect In Periodic Table Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This lecture is about shielding effect and screening effect in the periodic table. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the. Shielding is caused by the combination of. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Is Shielding Effect In Periodic Table Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. Hence, the nucleus has less grip on the. Shielding is caused by the combination of.. What Is Shielding Effect In Periodic Table.
From www.slideshare.net
Shielding Effect periotic table and preodicity of property PPT What Is Shielding Effect In Periodic Table Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Hence, the nucleus has less grip on the. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. Shielding is caused by the combination of.. What Is Shielding Effect In Periodic Table.
From www.allthescience.org
What Is the Shielding Effect? (with pictures) What Is Shielding Effect In Periodic Table The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and. What Is Shielding Effect In Periodic Table.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Shielding Effect In Periodic Table In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Hence, the nucleus has less grip on the. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. Shielding is caused by. What Is Shielding Effect In Periodic Table.
From www.slideshare.net
Chapter 6 Periodic Table What Is Shielding Effect In Periodic Table Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This is because of shielding, or simply the electrons closest to the nucleus. What Is Shielding Effect In Periodic Table.
From www.slideserve.com
PPT Drill 6 11/11 & 11/12/13 PowerPoint Presentation, free download What Is Shielding Effect In Periodic Table The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and. What Is Shielding Effect In Periodic Table.
From www.slideshare.net
Periodic trends What Is Shielding Effect In Periodic Table This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the. This lecture is about shielding effect and. What Is Shielding Effect In Periodic Table.
From www.youtube.com
What is a shielding effect ? Its Trends in periodic table Chemistry What Is Shielding Effect In Periodic Table This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This lecture is about shielding effect and screening effect in the periodic. What Is Shielding Effect In Periodic Table.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID118383 What Is Shielding Effect In Periodic Table Hence, the nucleus has less grip on the. This lecture is about shielding effect and screening effect in the periodic table. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the. What Is Shielding Effect In Periodic Table.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Shielding Effect In Periodic Table Hence, the nucleus has less grip on the. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This lecture is about shielding effect and screening effect in the periodic table. The shielding effect describes the balance between the pull of the protons on valence. What Is Shielding Effect In Periodic Table.
From www.youtube.com
what is shielding effect and it's effect in periodic table What Is Shielding Effect In Periodic Table Hence, the nucleus has less grip on the. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This lecture is about shielding. What Is Shielding Effect In Periodic Table.
From www.youtube.com
SHIELDING EFFECT & SLATER'S RULE/Periodic Table7H YouTube What Is Shielding Effect In Periodic Table This lecture is about shielding effect and screening effect in the periodic table. Shielding is caused by the combination of. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then. What Is Shielding Effect In Periodic Table.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Shielding Effect In Periodic Table Shielding is caused by the combination of. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Hence, the nucleus has less grip on the. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the. What Is Shielding Effect In Periodic Table.
From www.youtube.com
Shielding Effect,effective nuclear charge, Trend in Periodic Table What Is Shielding Effect In Periodic Table The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Shielding is caused by the combination of. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. Hence, the nucleus has less grip on the.. What Is Shielding Effect In Periodic Table.
From www.slideserve.com
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free What Is Shielding Effect In Periodic Table This lecture is about shielding effect and screening effect in the periodic table. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the. What Is Shielding Effect In Periodic Table.
From www.youtube.com
what is ionization energy?Shielding effect Trend in periodic table What Is Shielding Effect In Periodic Table This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Shielding is caused by the combination of. In this section, we explore one model for. What Is Shielding Effect In Periodic Table.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is Shielding Effect In Periodic Table Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount. What Is Shielding Effect In Periodic Table.
From www.youtube.com
Trend of Shielding Effect in Periodic Table, Chemistry Lecture Sabaq What Is Shielding Effect In Periodic Table In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This lecture is about shielding effect and screening effect in the periodic table. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons.. What Is Shielding Effect In Periodic Table.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Shielding Effect In Periodic Table The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Shielding is caused by the combination of. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. Hence, the nucleus has less grip on the.. What Is Shielding Effect In Periodic Table.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Is Shielding Effect In Periodic Table Hence, the nucleus has less grip on the. Shielding is caused by the combination of. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the. What Is Shielding Effect In Periodic Table.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Shielding Effect In Periodic Table Hence, the nucleus has less grip on the. This lecture is about shielding effect and screening effect in the periodic table. Shielding is caused by the combination of. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. This is because of shielding, or simply the electrons closest. What Is Shielding Effect In Periodic Table.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID What Is Shielding Effect In Periodic Table The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Shielding is caused by the combination of. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This is because of shielding, or. What Is Shielding Effect In Periodic Table.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Shielding Effect In Periodic Table Shielding is caused by the combination of. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This lecture is about shielding effect. What Is Shielding Effect In Periodic Table.
From www.chemistrylearner.com
Periodic Trends Definition and Properties What Is Shielding Effect In Periodic Table Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons. What Is Shielding Effect In Periodic Table.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Shielding Effect In Periodic Table This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. This lecture is about shielding effect and screening effect in the periodic table. Shielding is. What Is Shielding Effect In Periodic Table.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Shielding Effect In Periodic Table The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. This lecture is about shielding effect and screening effect in the periodic table. Hence, the nucleus has less grip on the. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then. What Is Shielding Effect In Periodic Table.
From www.youtube.com
periodic table nuclear charge and shielding YouTube What Is Shielding Effect In Periodic Table This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This lecture is about shielding effect and screening effect in the periodic. What Is Shielding Effect In Periodic Table.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Shielding Effect In Periodic Table Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Hence, the nucleus has less grip on the. This lecture is about shielding effect and screening effect in the periodic table. Shielding is caused by the combination of. The shielding effect describes the balance between the pull of. What Is Shielding Effect In Periodic Table.
From www.youtube.com
Shielding Effect in the Periodic Table Chemistry YouTube What Is Shielding Effect In Periodic Table This lecture is about shielding effect and screening effect in the periodic table. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge.. What Is Shielding Effect In Periodic Table.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Is Shielding Effect In Periodic Table This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. Hence, the nucleus has less grip on the. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Shielding refers to the. What Is Shielding Effect In Periodic Table.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Shielding Effect In Periodic Table The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Hence, the nucleus has less grip on the. Shielding refers to the core. What Is Shielding Effect In Periodic Table.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation ID2834450 What Is Shielding Effect In Periodic Table This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons. What Is Shielding Effect In Periodic Table.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download What Is Shielding Effect In Periodic Table Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. The shielding effect describes the balance between the pull of the protons on. What Is Shielding Effect In Periodic Table.
From www.slideshare.net
Shielding Effect periotic table and preodicity of property PPT What Is Shielding Effect In Periodic Table This lecture is about shielding effect and screening effect in the periodic table. Shielding is caused by the combination of. Hence, the nucleus has less grip on the. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. This is because of shielding, or simply the electrons closest. What Is Shielding Effect In Periodic Table.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is Shielding Effect In Periodic Table In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. This is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. Shielding refers to the core electrons repelling the outer electrons, which lowers. What Is Shielding Effect In Periodic Table.