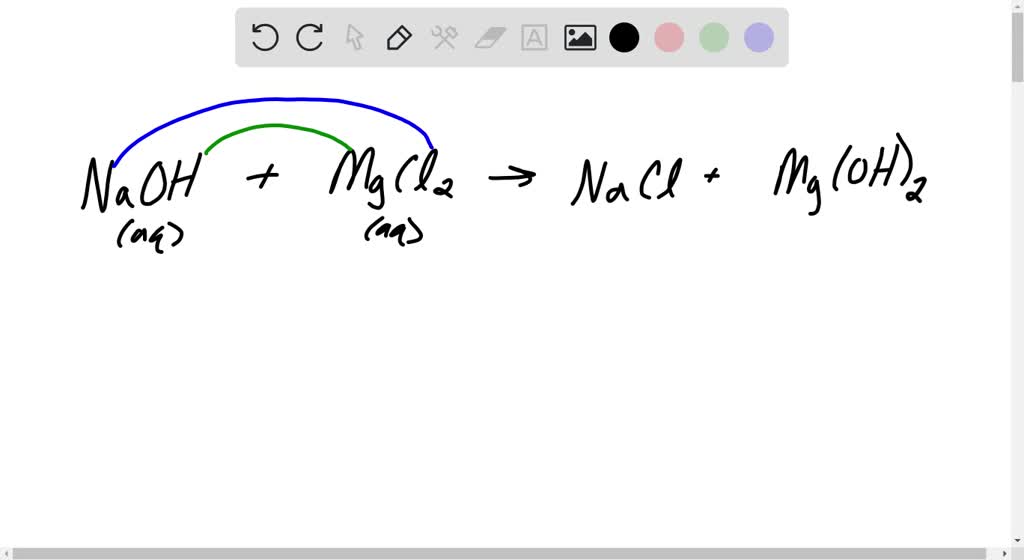
Magnesium Chloride And Potassium Hydroxide Precipitate . koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. Lime (calcium hydroxide) or dolomite is added to seawater to. — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. Find guidelines for solubility of ionic compounds, examples and exercises. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. — objectivewhat happens when magnesium chloride (mgcl2). learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. — precipitation of magnesium hydroxide: learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility.
from www.numerade.com
mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility. — precipitation of magnesium hydroxide: Find guidelines for solubility of ionic compounds, examples and exercises. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. Lime (calcium hydroxide) or dolomite is added to seawater to. — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. — objectivewhat happens when magnesium chloride (mgcl2).
SOLVED What will cause more Magnesium to be dissolved in a solution of
Magnesium Chloride And Potassium Hydroxide Precipitate Lime (calcium hydroxide) or dolomite is added to seawater to. learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. — objectivewhat happens when magnesium chloride (mgcl2). Lime (calcium hydroxide) or dolomite is added to seawater to. Find guidelines for solubility of ionic compounds, examples and exercises. — precipitation of magnesium hydroxide: learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide.
From www.youtube.com
Does Potassium hydroxide (KOH) and Iron(III) chloride (FeCl3) form a Magnesium Chloride And Potassium Hydroxide Precipitate a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility. koh + mgcl2. Magnesium Chloride And Potassium Hydroxide Precipitate.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Magnesium Chloride And Potassium Hydroxide Precipitate — objectivewhat happens when magnesium chloride (mgcl2). Find guidelines for solubility of ionic compounds, examples and exercises. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. — precipitation of magnesium. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.youtube.com
NaOH + MgSO4 Reaction & Precipitate Blossoms 💮 YouTube Magnesium Chloride And Potassium Hydroxide Precipitate — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. Find guidelines for solubility of ionic compounds, examples and exercises. learn how to predict the solubility and formation of precipitates of. Magnesium Chloride And Potassium Hydroxide Precipitate.
From hxeyernyc.blob.core.windows.net
Magnesium Bromide And Potassium Hydroxide Precipitate at Tabitha Moore blog Magnesium Chloride And Potassium Hydroxide Precipitate — precipitation of magnesium hydroxide: Find guidelines for solubility of ionic compounds, examples and exercises. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. a precipitate. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED Will potassium hydroxide and magnesium bromide form a Magnesium Chloride And Potassium Hydroxide Precipitate Find guidelines for solubility of ionic compounds, examples and exercises. — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. learn how to predict the solubility and formation of precipitates of common inorganic compounds. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Magnesium Chloride And Potassium Hydroxide Precipitate a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. Find guidelines for solubility of ionic compounds, examples and exercises. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. learn how to predict the solubility and formation of precipitates of common. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.sciencephoto.com
Magnesium Hydroxide Precipitate Stock Image C027/9874 Science Magnesium Chloride And Potassium Hydroxide Precipitate mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility. — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. Find guidelines for solubility of. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED 'er on test tube hydroxide and magnesium nitrate were mixed Magnesium Chloride And Potassium Hydroxide Precipitate Lime (calcium hydroxide) or dolomite is added to seawater to. — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction. Magnesium Chloride And Potassium Hydroxide Precipitate.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Magnesium Chloride And Potassium Hydroxide Precipitate Lime (calcium hydroxide) or dolomite is added to seawater to. — precipitation of magnesium hydroxide: Find guidelines for solubility of ionic compounds, examples and exercises. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one. Magnesium Chloride And Potassium Hydroxide Precipitate.
From ar.inspiredpencil.com
Magnesium And Water Reaction Magnesium Chloride And Potassium Hydroxide Precipitate — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. — precipitation of magnesium hydroxide: koh. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.youtube.com
How to Write the Net Ionic Equation for MgCl2 + KOH = Mg(OH)2 + KCl Magnesium Chloride And Potassium Hydroxide Precipitate mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. Find guidelines for solubility of ionic compounds, examples and exercises. koh + mgcl2 = kcl + mg(oh)2 is a double. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Chloride And Potassium Hydroxide Precipitate Lime (calcium hydroxide) or dolomite is added to seawater to. — precipitation of magnesium hydroxide: — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. learn how to identify, predict. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED What will cause more Magnesium to be dissolved in a solution of Magnesium Chloride And Potassium Hydroxide Precipitate Find guidelines for solubility of ionic compounds, examples and exercises. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. — objectivewhat happens when magnesium chloride (mgcl2). Lime (calcium hydroxide) or dolomite is added to seawater to. — precipitation of magnesium hydroxide: koh + mgcl2 = kcl +. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED An aqueous solution of magnesium chloride, MgCl2, is added to Magnesium Chloride And Potassium Hydroxide Precipitate — precipitation of magnesium hydroxide: mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. learn how to predict the solubility and formation of precipitates of common inorganic compounds using. Magnesium Chloride And Potassium Hydroxide Precipitate.
From dxoygaprp.blob.core.windows.net
What Is Molecular And Ionic at Patricia Ledbetter blog Magnesium Chloride And Potassium Hydroxide Precipitate mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. — objectivewhat happens when magnesium chloride (mgcl2). a precipitate forms when a solution of magnesium chloride is. Magnesium Chloride And Potassium Hydroxide Precipitate.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Magnesium Chloride And Potassium Hydroxide Precipitate a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. Find guidelines for solubility. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED A student determines the magnesium content of a solution by Magnesium Chloride And Potassium Hydroxide Precipitate learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride.. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
Molecules, Compounds, and Chemical Equations Ch… Magnesium Chloride And Potassium Hydroxide Precipitate — objectivewhat happens when magnesium chloride (mgcl2). — precipitation of magnesium hydroxide: Find guidelines for solubility of ionic compounds, examples and exercises. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where. Magnesium Chloride And Potassium Hydroxide Precipitate.
From pixels.com
Magnesium Hydroxide Precipitate Photograph by Andrew Lambert Magnesium Chloride And Potassium Hydroxide Precipitate a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. Find guidelines for. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.youtube.com
Precipitation Reaction Unveiled Calcium Chloride and Potassium Magnesium Chloride And Potassium Hydroxide Precipitate — precipitation of magnesium hydroxide: — objectivewhat happens when magnesium chloride (mgcl2). mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. Lime (calcium hydroxide) or dolomite is added to seawater to. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where. Magnesium Chloride And Potassium Hydroxide Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Magnesium Chloride And Potassium Hydroxide Precipitate learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. Lime (calcium hydroxide) or dolomite is added to seawater to. — objectivewhat happens when magnesium chloride (mgcl2). learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility. koh + mgcl2 = kcl. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.sciencephoto.com
Magnesium Hydroxide Precipitate Stock Image C027/9873 Science Magnesium Chloride And Potassium Hydroxide Precipitate Lime (calcium hydroxide) or dolomite is added to seawater to. learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. learn how to identify, predict and apply precipitation reactions, a subclass of. Magnesium Chloride And Potassium Hydroxide Precipitate.
From melscience.com
Reactions of potassium and potassium hydroxide MEL Chemistry Magnesium Chloride And Potassium Hydroxide Precipitate a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. — precipitation of magnesium hydroxide: mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Magnesium Chloride And Potassium Hydroxide Precipitate Find guidelines for solubility of ionic compounds, examples and exercises. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. Lime (calcium hydroxide) or dolomite is added to seawater to. — objectivewhat happens when magnesium chloride (mgcl2). — learn how to identify and predict precipitation reactions, which are reactions that. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.mdpi.com
Sustainability Free FullText Is KStruvite Precipitation a Magnesium Chloride And Potassium Hydroxide Precipitate Lime (calcium hydroxide) or dolomite is added to seawater to. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. — precipitation of magnesium hydroxide: koh + mgcl2 = kcl + mg(oh)2. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Magnesium Chloride And Potassium Hydroxide Precipitate — precipitation of magnesium hydroxide: — objectivewhat happens when magnesium chloride (mgcl2). mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. Lime (calcium hydroxide) or dolomite is added to seawater to. — learn how to identify and predict precipitation reactions, which are reactions that yield. Magnesium Chloride And Potassium Hydroxide Precipitate.
From hxeyernyc.blob.core.windows.net
Magnesium Bromide And Potassium Hydroxide Precipitate at Tabitha Moore blog Magnesium Chloride And Potassium Hydroxide Precipitate koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. Lime (calcium hydroxide) or dolomite is added to seawater to. Find guidelines for solubility of ionic compounds, examples and exercises. . Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.coursehero.com
[Solved] Write the overall molecular equation for the reaction of Magnesium Chloride And Potassium Hydroxide Precipitate Lime (calcium hydroxide) or dolomite is added to seawater to. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. — precipitation of magnesium hydroxide: learn how to predict the. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.studyxapp.com
1340 magnesium chloride and sodium hydroxide react form magnesium Magnesium Chloride And Potassium Hydroxide Precipitate Find guidelines for solubility of ionic compounds, examples and exercises. learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility. — objectivewhat happens when magnesium chloride (mgcl2). — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. mgcl2 + koh = mg(oh)2 + kcl. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.researchgate.net
(PDF) Sorption of Phosphate on Douglas Fir Biochar Treated with Magnesium Chloride And Potassium Hydroxide Precipitate — learn how to identify and predict precipitation reactions, which are reactions that yield insoluble products. Find guidelines for solubility of ionic compounds, examples and exercises. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. — precipitation of magnesium hydroxide: a precipitate forms when a solution. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.youtube.com
Potassium phosphate + Magnesium chloride (balanced equation) YouTube Magnesium Chloride And Potassium Hydroxide Precipitate learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. — objectivewhat. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.nagwa.com
Question Video Identifying a Precipitating Agent for the Gravimetric Magnesium Chloride And Potassium Hydroxide Precipitate — objectivewhat happens when magnesium chloride (mgcl2). — precipitation of magnesium hydroxide: Find guidelines for solubility of ionic compounds, examples and exercises. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.bartleby.com
Answered Complete the table below by deciding… bartleby Magnesium Chloride And Potassium Hydroxide Precipitate a precipitate forms when a solution of magnesium chloride is mixed with a solution of potassium hydroxide. — objectivewhat happens when magnesium chloride (mgcl2). Lime (calcium hydroxide) or dolomite is added to seawater to. koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction where two moles of aqueous potassium. — precipitation of. Magnesium Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED Potassium hydroxide is used to precipitate each of the cations Magnesium Chloride And Potassium Hydroxide Precipitate Lime (calcium hydroxide) or dolomite is added to seawater to. Find guidelines for solubility of ionic compounds, examples and exercises. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products.. Magnesium Chloride And Potassium Hydroxide Precipitate.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Magnesium Chloride And Potassium Hydroxide Precipitate learn how to predict the solubility and formation of precipitates of common inorganic compounds using solubility. mgcl2 + koh = mg(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. — objectivewhat happens when magnesium chloride (mgcl2). koh + mgcl2 = kcl + mg(oh)2 is a double displacement (metathesis) reaction. Magnesium Chloride And Potassium Hydroxide Precipitate.