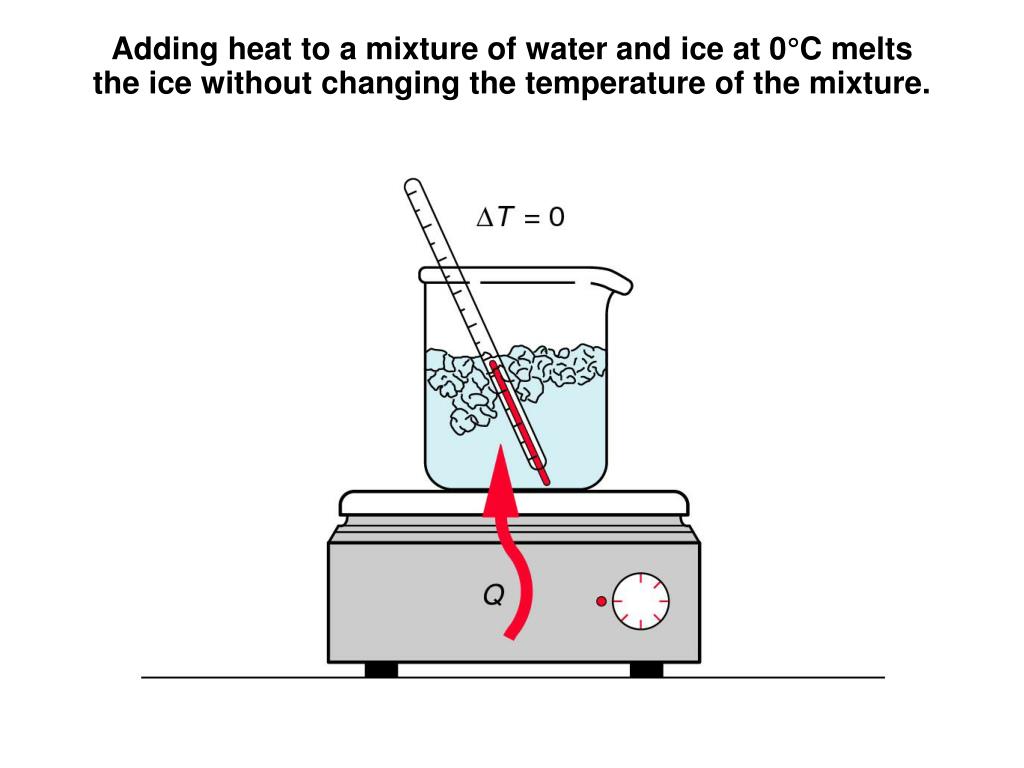
What Is The Role Of Energy When Ice Melts . Heat is transferred from the soda to the ice for melting. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the temperature of this water rises. Heat is transferred from the soda to the ice for melting. the ice cubes are at the melting temperature of 0oc. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules in place while in its solid form. Melting of ice occurs in two steps: the ice cubes are at the melting temperature of 0ºc. the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. Melting of ice occurs in two steps: As the ice melts, its temperature does not rise. as the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more.
from www.slideserve.com
the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. Heat is transferred from the soda to the ice for melting. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules in place while in its solid form. As the ice melts, its temperature does not rise. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. Melting of ice occurs in two steps: Heat is transferred from the soda to the ice for melting. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. Melting of ice occurs in two steps: the ice cubes are at the melting temperature of 0oc.
PPT Pascal’s Principle PowerPoint Presentation, free download ID
What Is The Role Of Energy When Ice Melts in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. the ice cubes are at the melting temperature of 0ºc. the ice cubes are at the melting temperature of 0oc. Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. Melting of ice occurs in two steps: the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. Melting of ice occurs in two steps: as the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the temperature of this water rises. Heat is transferred from the soda to the ice for melting. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. As the ice melts, its temperature does not rise. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules in place while in its solid form. Heat is transferred from the soda to the ice for melting. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them.
From edenapp.com
Types of Ice Melt Eden Lawn Care and Snow Removal What Is The Role Of Energy When Ice Melts Melting of ice occurs in two steps: the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. the ice cubes are at the melting temperature of 0oc. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the temperature of this. What Is The Role Of Energy When Ice Melts.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science What Is The Role Of Energy When Ice Melts Heat is transferred from the soda to the ice for melting. Melting of ice occurs in two steps: Heat is transferred from the soda to the ice for melting. as the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more. the heat energy causes the ice molecules. What Is The Role Of Energy When Ice Melts.
From www.pinterest.com
What Makes Ice Melt Faster? What Is The Role Of Energy When Ice Melts the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. the ice cubes are at the melting temperature of 0oc. Heat is transferred from the soda to the ice for melting. in the case of ice melting under atmospheric pressure, the volume contraction. What Is The Role Of Energy When Ice Melts.
From studylib.net
What Substance Makes Ice Melt The Fastest? What Is The Role Of Energy When Ice Melts Heat is transferred from the soda to the ice for melting. As the ice melts, its temperature does not rise. the ice cubes are at the melting temperature of 0oc. Heat is transferred from the soda to the ice for melting. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds. What Is The Role Of Energy When Ice Melts.
From thebackyardpros.com
At What Temperature Does Ice Melt Work? (10 Examples) The Backyard Pros What Is The Role Of Energy When Ice Melts the ice cubes are at the melting temperature of 0ºc. the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. Heat is transferred from the soda to the ice for melting. the heat energy causes the ice molecules to gain kinetic energy and. What Is The Role Of Energy When Ice Melts.
From courses.lumenlearning.com
Some Basic Definitions Introductory Chemistry What Is The Role Of Energy When Ice Melts Melting of ice occurs in two steps: Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. Heat is transferred from the soda to the ice for melting. in the case of ice melting under atmospheric pressure, the volume contraction means. What Is The Role Of Energy When Ice Melts.
From researchfeatures.com
Exploring the links between melting ice and ecosystems What Is The Role Of Energy When Ice Melts Heat is transferred from the soda to the ice for melting. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules in place while in its solid form. As the ice melts, its temperature does not rise. Heat is transferred from the soda to the. What Is The Role Of Energy When Ice Melts.
From www.slideserve.com
PPT Chapter 17 Free Energy and Thermodynamics PowerPoint Presentation What Is The Role Of Energy When Ice Melts the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. First the phase change occurs and solid (ice) transforms into liquid. What Is The Role Of Energy When Ice Melts.
From hs.envirotechservices.com
What is the Best Value Ice Melt? What Is The Role Of Energy When Ice Melts the ice cubes are at the melting temperature of 0ºc. As the ice melts, its temperature does not rise. Melting of ice occurs in two steps: Heat is transferred from the soda to the ice for melting. the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to. What Is The Role Of Energy When Ice Melts.
From cedqzwdi.blob.core.windows.net
What Is The Melting Point Of Snow at Arthur Taylor blog What Is The Role Of Energy When Ice Melts the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. the ice cubes are at the melting temperature of 0ºc. as the temperature of the. What Is The Role Of Energy When Ice Melts.
From www.metoffice.gov.uk
Sea ice in the climate system Met Office What Is The Role Of Energy When Ice Melts Heat is transferred from the soda to the ice for melting. Heat is transferred from the soda to the ice for melting. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. Energy is required to change the phase of a substance, such as the energy to break the. What Is The Role Of Energy When Ice Melts.
From www.slideserve.com
PPT What Substance Makes Ice Melt The Fastest? PowerPoint What Is The Role Of Energy When Ice Melts Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. As the ice melts, its temperature does not rise. the heat energy causes. What Is The Role Of Energy When Ice Melts.
From www.lihpao.com
What Makes Ice Melt Fastest? Exploring the Science Behind Melting Ice What Is The Role Of Energy When Ice Melts in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. the ice cubes are at the melting temperature of 0ºc. eventually, when the ice has warmed to 0°c, the. What Is The Role Of Energy When Ice Melts.
From lessondbcompactest.z5.web.core.windows.net
How Does Sugar Melt Ice What Is The Role Of Energy When Ice Melts in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. the ice cubes are at the melting temperature of 0ºc. Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. Melting of ice. What Is The Role Of Energy When Ice Melts.
From sciencenotes.org
Regelation of Ice in Physics and Chemistry What Is The Role Of Energy When Ice Melts First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the temperature of this water rises. Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. Melting of ice occurs in two steps: Heat is. What Is The Role Of Energy When Ice Melts.
From www.slideserve.com
PPT Energy and Chemical Change PowerPoint Presentation, free download What Is The Role Of Energy When Ice Melts Heat is transferred from the soda to the ice for melting. Melting of ice occurs in two steps: the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal. What Is The Role Of Energy When Ice Melts.
From blog.iceslicer.com
How to Use Ice Melt Responsibly What Is The Role Of Energy When Ice Melts as the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. What Is The Role Of Energy When Ice Melts.
From circuitdiagramlows.z22.web.core.windows.net
Phase Change Diagram For Water What Is The Role Of Energy When Ice Melts as the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more. Melting of ice occurs in two steps: Heat is transferred from the soda to the ice for melting. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. Energy. What Is The Role Of Energy When Ice Melts.
From www.lihpao.com
What Melts Ice the Fastest? Exploring Different Methods in a Science What Is The Role Of Energy When Ice Melts eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules in place while in its solid form. Melting of ice occurs in two steps: As the ice melts, its temperature does not rise. in the case of ice melting under atmospheric pressure, the volume. What Is The Role Of Energy When Ice Melts.
From www.slideserve.com
PPT Pascal’s Principle PowerPoint Presentation, free download ID What Is The Role Of Energy When Ice Melts in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. the ice cubes are at the melting temperature of 0ºc. Heat is transferred from the soda to the ice for. What Is The Role Of Energy When Ice Melts.
From www.slideshare.net
Heat What Is The Role Of Energy When Ice Melts Melting of ice occurs in two steps: eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules in place while in its solid form. the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed. What Is The Role Of Energy When Ice Melts.
From study.com
Polar Ice Caps Melting Causes & Impacts Lesson What Is The Role Of Energy When Ice Melts the ice cubes are at the melting temperature of 0oc. As the ice melts, its temperature does not rise. the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster,. What Is The Role Of Energy When Ice Melts.
From socratic.org
What happens to the energy of its molecules as ice melts into What Is The Role Of Energy When Ice Melts the ice cubes are at the melting temperature of 0oc. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules in place while in its solid form. the amount of energy need to melt a kilogram of ice (334 kj) is the same. What Is The Role Of Energy When Ice Melts.
From www.numerade.com
SOLVED The loss of Arctic ice is accelerating as a result of a form of What Is The Role Of Energy When Ice Melts Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. the ice cubes are at the melting temperature of 0ºc. as the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and. What Is The Role Of Energy When Ice Melts.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) What Is The Role Of Energy When Ice Melts eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules in place while in its solid form. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. the ice cubes are at the. What Is The Role Of Energy When Ice Melts.
From www.sciencelearn.org.nz
Heat and change of state — Science Learning Hub What Is The Role Of Energy When Ice Melts Heat is transferred from the soda to the ice for melting. Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. the ice cubes are at the melting temperature of 0oc. as the temperature of the ice increases, the water. What Is The Role Of Energy When Ice Melts.
From www.haikudeck.com
Ice Melt by Sean Mulhern What Is The Role Of Energy When Ice Melts in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. As the ice melts, its temperature does not rise. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. Energy is required to change the phase of a substance, such as. What Is The Role Of Energy When Ice Melts.
From www.scienceabc.com
What Is The First Law Of Thermodynamics? » ScienceABC What Is The Role Of Energy When Ice Melts as the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more. Heat is transferred from the soda to the ice for melting. the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. . What Is The Role Of Energy When Ice Melts.
From ian.umces.edu
Experiment Demonstrating Melting Ice Media Library Integration and What Is The Role Of Energy When Ice Melts the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. Melting of ice occurs in two steps: eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules in place while in its solid form.. What Is The Role Of Energy When Ice Melts.
From apollo.nvu.vsc.edu
Latent Heats sublimation and deposition What Is The Role Of Energy When Ice Melts Energy is required to change the phase of a substance, such as the energy to break the bonds between molecules in a block of ice so it may. as the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more. the ice cubes are at the melting temperature. What Is The Role Of Energy When Ice Melts.
From slideplayer.com
Temperature, Heat, and Expansion ppt download What Is The Role Of Energy When Ice Melts the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. As the ice melts, its temperature does not rise. Melting of ice occurs in two steps: in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. the ice cubes are. What Is The Role Of Energy When Ice Melts.
From www.vectorstock.com
Diagram showing how ice melts Royalty Free Vector Image What Is The Role Of Energy When Ice Melts Heat is transferred from the soda to the ice for melting. Heat is transferred from the soda to the ice for melting. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. Melting of ice occurs in two steps: eventually, when the ice has warmed to 0°c, the. What Is The Role Of Energy When Ice Melts.
From saylordotorg.github.io
Chemical Thermodynamics What Is The Role Of Energy When Ice Melts Heat is transferred from the soda to the ice for melting. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. Melting of ice occurs in two steps: eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that. What Is The Role Of Energy When Ice Melts.
From lessonlibincohesion.z22.web.core.windows.net
Ice Melting Science Experiments What Is The Role Of Energy When Ice Melts the ice cubes are at the melting temperature of 0ºc. the heat energy causes the ice molecules to gain kinetic energy and vibrate faster, breaking the bonds that hold them. the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. Heat is transferred. What Is The Role Of Energy When Ice Melts.
From sites.google.com
Melting of Polar Ice Caps MoGlobalClimate What Is The Role Of Energy When Ice Melts in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. the amount of energy need to melt a kilogram of ice (334 kj) is the same amount of energy needed to raise the temperature. Melting of ice occurs in two steps: As the ice melts, its temperature does not rise. . What Is The Role Of Energy When Ice Melts.