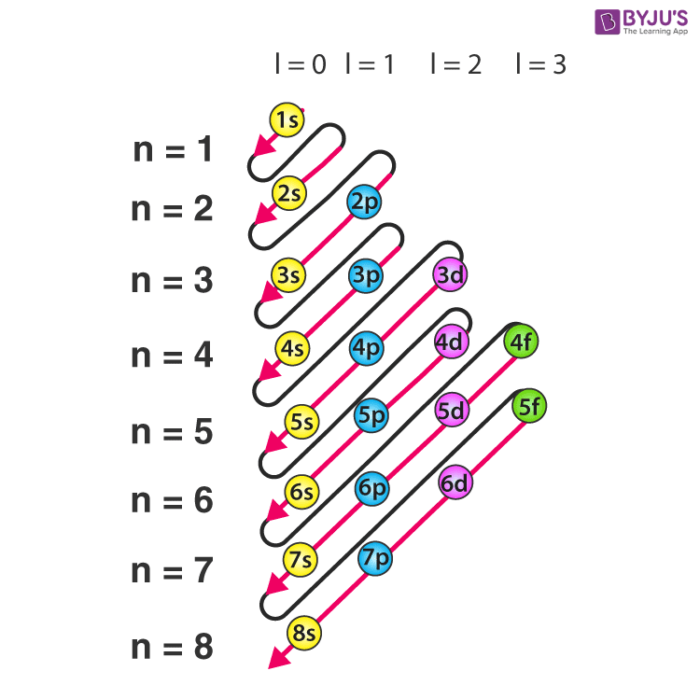
Formula For Writing Electronic Configuration . 3 rules of writing electronic configurations of atoms 1. What is the electron configuration and orbital diagram of: The maximum number of electrons in the main quantum. The added electron will always occupy the orbital with the lowest energy first. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. Describe the major concepts (hunds, pauli.etc.) and explain why. Electronic configuration or electron configuration of orbitals takes place according to the following rules. First, write out the electron. Each orbital can hold a maximum of two electrons of opposite spins. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. By the end of this section, you will be able to: The electron configuration of an atom is written with the help of subshell labels. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy.
from byjus.com
Describe the major concepts (hunds, pauli.etc.) and explain why. The electron configuration of an atom is written with the help of subshell labels. Each orbital can hold a maximum of two electrons of opposite spins. First, write out the electron. 3 rules of writing electronic configurations of atoms 1. What is the electron configuration and orbital diagram of: The maximum number of electrons in the main quantum. By the end of this section, you will be able to: Electronic configuration or electron configuration of orbitals takes place according to the following rules. The added electron will always occupy the orbital with the lowest energy first.
Electron Configuration Detailed Explanation, Filling of orbital
Formula For Writing Electronic Configuration Describe the major concepts (hunds, pauli.etc.) and explain why. Electronic configuration or electron configuration of orbitals takes place according to the following rules. The electron configuration of an atom is written with the help of subshell labels. Describe the major concepts (hunds, pauli.etc.) and explain why. First, write out the electron. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. 3 rules of writing electronic configurations of atoms 1. The maximum number of electrons in the main quantum. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. By the end of this section, you will be able to: These labels contain the shell number (given by the principal quantum number), the subshell name (given by. The added electron will always occupy the orbital with the lowest energy first. Each orbital can hold a maximum of two electrons of opposite spins. What is the electron configuration and orbital diagram of:
From www.pinterest.com
Electron Configuration of Atoms gen chem refresher for organic Formula For Writing Electronic Configuration 3 rules of writing electronic configurations of atoms 1. Each orbital can hold a maximum of two electrons of opposite spins. The added electron will always occupy the orbital with the lowest energy first. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. Describe the major. Formula For Writing Electronic Configuration.
From brunofuga.adv.br
Write Electronic Configuration Of Element, 54 OFF Formula For Writing Electronic Configuration What is the electron configuration and orbital diagram of: These labels contain the shell number (given by the principal quantum number), the subshell name (given by. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. The electron configuration of an atom is written with the help. Formula For Writing Electronic Configuration.
From www.slideserve.com
PPT Electron Configurations PowerPoint Presentation, free download Formula For Writing Electronic Configuration Each orbital can hold a maximum of two electrons of opposite spins. 3 rules of writing electronic configurations of atoms 1. First, write out the electron. Electronic configuration or electron configuration of orbitals takes place according to the following rules. Describe the major concepts (hunds, pauli.etc.) and explain why. In your own words describe how to write an electron configuration. Formula For Writing Electronic Configuration.
From www.pinterest.com
Using the Electron Configuration Chart Electron configuration Formula For Writing Electronic Configuration The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Each orbital can hold a maximum of two electrons of opposite spins. The added electron will always occupy the orbital with the lowest energy first. The electron configuration of an atom is. Formula For Writing Electronic Configuration.
From persepolisthesis.web.fc2.com
How to write electron configuration of atoms Formula For Writing Electronic Configuration These labels contain the shell number (given by the principal quantum number), the subshell name (given by. The electron configuration of an atom is written with the help of subshell labels. The added electron will always occupy the orbital with the lowest energy first. By the end of this section, you will be able to: First, write out the electron.. Formula For Writing Electronic Configuration.
From www.vedantu.com
Electronic Configuration Antimony Learn Important Terms and Concepts Formula For Writing Electronic Configuration In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. The electron configuration of an atom is written with the help of subshell labels. The maximum number of electrons in the main quantum. 3 rules of writing electronic configurations of atoms 1. These labels contain the shell. Formula For Writing Electronic Configuration.
From www.youtube.com
How to Write Electron Configurations and Orbital Diagrams (General Formula For Writing Electronic Configuration What is the electron configuration and orbital diagram of: 3 rules of writing electronic configurations of atoms 1. The maximum number of electrons in the main quantum. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. These labels contain the shell number (given by the principal. Formula For Writing Electronic Configuration.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Formula For Writing Electronic Configuration The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Electronic configuration or electron configuration of orbitals takes place according to the following rules. The electron configuration of an atom is written with the help of subshell labels. These labels contain the. Formula For Writing Electronic Configuration.
From byjus.com
Electron Configuration Detailed Explanation, Filling of orbital Formula For Writing Electronic Configuration What is the electron configuration and orbital diagram of: The electron configuration of an atom is written with the help of subshell labels. First, write out the electron. Each orbital can hold a maximum of two electrons of opposite spins. In your own words describe how to write an electron configuration and why it is an important skill in the. Formula For Writing Electronic Configuration.
From www.slideserve.com
PPT Unit 3 Atomic Structure PowerPoint Presentation, free download Formula For Writing Electronic Configuration Each orbital can hold a maximum of two electrons of opposite spins. The electron configuration of an atom is written with the help of subshell labels. The added electron will always occupy the orbital with the lowest energy first. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. 3 rules of writing. Formula For Writing Electronic Configuration.
From www.youtube.com
Trick to write electronic configuration of Lanthanides YouTube Formula For Writing Electronic Configuration In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. Each orbital can hold a maximum of two electrons of opposite spins. By the end of this section, you will be able to: First, write out the electron. The maximum number of electrons in the main quantum.. Formula For Writing Electronic Configuration.
From www.youtube.com
Using the Diagonal Rule to Write Electron Configurations YouTube Formula For Writing Electronic Configuration Describe the major concepts (hunds, pauli.etc.) and explain why. 3 rules of writing electronic configurations of atoms 1. The maximum number of electrons in the main quantum. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. First, write out the electron.. Formula For Writing Electronic Configuration.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write Formula For Writing Electronic Configuration The added electron will always occupy the orbital with the lowest energy first. The maximum number of electrons in the main quantum. The electron configuration of an atom is written with the help of subshell labels. Describe the major concepts (hunds, pauli.etc.) and explain why. By the end of this section, you will be able to: Each orbital can hold. Formula For Writing Electronic Configuration.
From www.youtube.com
Electron configuration spdf notation Part 2 YouTube Formula For Writing Electronic Configuration 3 rules of writing electronic configurations of atoms 1. First, write out the electron. Describe the major concepts (hunds, pauli.etc.) and explain why. Each orbital can hold a maximum of two electrons of opposite spins. The added electron will always occupy the orbital with the lowest energy first. In your own words describe how to write an electron configuration and. Formula For Writing Electronic Configuration.
From byjus.com
How to write the electronic configuration for a given element? Formula For Writing Electronic Configuration The maximum number of electrons in the main quantum. The added electron will always occupy the orbital with the lowest energy first. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. In your own words describe how to write an electron. Formula For Writing Electronic Configuration.
From www.pinterest.com
Writing Electron Configurations Using Only the Periodic Table Formula For Writing Electronic Configuration The maximum number of electrons in the main quantum. 3 rules of writing electronic configurations of atoms 1. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Electronic configuration or electron configuration of orbitals takes place according to the following rules.. Formula For Writing Electronic Configuration.
From www.youtube.com
How to Write the Electron Configuration of an Element Study Chemistry Formula For Writing Electronic Configuration Each orbital can hold a maximum of two electrons of opposite spins. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. First, write out the electron. The maximum number of electrons in the main quantum. Describe the major concepts (hunds, pauli.etc.) and explain why. The electron configuration of an atom is written. Formula For Writing Electronic Configuration.
From vanceknoehampton.blogspot.com
Electronic Configuration of Elements VanceknoeHampton Formula For Writing Electronic Configuration The maximum number of electrons in the main quantum. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. First, write out the electron. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2. Formula For Writing Electronic Configuration.
From printablelibixtle.z21.web.core.windows.net
Writing Out Electron Configurations Formula For Writing Electronic Configuration Each orbital can hold a maximum of two electrons of opposite spins. Describe the major concepts (hunds, pauli.etc.) and explain why. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. By the end of this section, you will be able to: The first integer, 1, gives us the principle energy level, the. Formula For Writing Electronic Configuration.
From www.youtube.com
Electronic Configuration of a Atom Atomic Structure Electronic Formula For Writing Electronic Configuration These labels contain the shell number (given by the principal quantum number), the subshell name (given by. Each orbital can hold a maximum of two electrons of opposite spins. Describe the major concepts (hunds, pauli.etc.) and explain why. By the end of this section, you will be able to: First, write out the electron. The electron configuration of an atom. Formula For Writing Electronic Configuration.
From www.youtube.com
Electronic Configuration easy formulla Easy way to understand Formula For Writing Electronic Configuration The maximum number of electrons in the main quantum. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. First, write out the electron. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. Describe the major concepts (hunds, pauli.etc.). Formula For Writing Electronic Configuration.
From www.youtube.com
Aleks Writing the electron configuration of an atom using the periodic Formula For Writing Electronic Configuration By the end of this section, you will be able to: Electronic configuration or electron configuration of orbitals takes place according to the following rules. First, write out the electron. Describe the major concepts (hunds, pauli.etc.) and explain why. In your own words describe how to write an electron configuration and why it is an important skill in the study. Formula For Writing Electronic Configuration.
From www.youtube.com
Rules for writing Electronic configuration YouTube Formula For Writing Electronic Configuration The electron configuration of an atom is written with the help of subshell labels. Describe the major concepts (hunds, pauli.etc.) and explain why. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Each orbital can hold a maximum of two electrons. Formula For Writing Electronic Configuration.
From vanceknoehampton.blogspot.com
Electronic Configuration of Elements VanceknoeHampton Formula For Writing Electronic Configuration First, write out the electron. Each orbital can hold a maximum of two electrons of opposite spins. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. By the end of this section, you will be able to: In your own words describe how to write an electron configuration and why it is. Formula For Writing Electronic Configuration.
From worksheetstemplates.netlify.app
Electron Configuration Chart Calculator Formula For Writing Electronic Configuration Electronic configuration or electron configuration of orbitals takes place according to the following rules. What is the electron configuration and orbital diagram of: These labels contain the shell number (given by the principal quantum number), the subshell name (given by. The electron configuration of an atom is written with the help of subshell labels. The first integer, 1, gives us. Formula For Writing Electronic Configuration.
From byjus.com
Electronic Configuration How To Write Electron ConfigurationChemistry Formula For Writing Electronic Configuration The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. The electron configuration of an atom is written with the help of subshell labels. First, write out the electron. In your own words describe how to write an electron configuration and why. Formula For Writing Electronic Configuration.
From www.youtube.com
Science Tutorial Writing the Electron Configuration Subshell Notation Formula For Writing Electronic Configuration Each orbital can hold a maximum of two electrons of opposite spins. The electron configuration of an atom is written with the help of subshell labels. Describe the major concepts (hunds, pauli.etc.) and explain why. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. The added. Formula For Writing Electronic Configuration.
From chemwiki.ucdavis.edu
Electronic Configurations Chemwiki Formula For Writing Electronic Configuration The maximum number of electrons in the main quantum. By the end of this section, you will be able to: Describe the major concepts (hunds, pauli.etc.) and explain why. Each orbital can hold a maximum of two electrons of opposite spins. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. The first. Formula For Writing Electronic Configuration.
From topblogtenz.com
How to write Electron Configuration All methods + Examples Formula For Writing Electronic Configuration What is the electron configuration and orbital diagram of: Each orbital can hold a maximum of two electrons of opposite spins. The added electron will always occupy the orbital with the lowest energy first. First, write out the electron. 3 rules of writing electronic configurations of atoms 1. These labels contain the shell number (given by the principal quantum number),. Formula For Writing Electronic Configuration.
From www.youtube.com
How to write electronic configuration spdf orbitals. NCERT CBSE Formula For Writing Electronic Configuration Describe the major concepts (hunds, pauli.etc.) and explain why. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. First, write out the electron. Electronic configuration or electron configuration of orbitals takes place according to the following rules. The maximum number of electrons in the main quantum.. Formula For Writing Electronic Configuration.
From tex.stackexchange.com
chemistry Box and arrow notation of writing electron configuration Formula For Writing Electronic Configuration The maximum number of electrons in the main quantum. The electron configuration of an atom is written with the help of subshell labels. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. First, write out the electron. What is the electron configuration and orbital diagram of:. Formula For Writing Electronic Configuration.
From tex.stackexchange.com
chemistry Box and arrow notation of writing electron configuration Formula For Writing Electronic Configuration Electronic configuration or electron configuration of orbitals takes place according to the following rules. What is the electron configuration and orbital diagram of: First, write out the electron. The electron configuration of an atom is written with the help of subshell labels. Each orbital can hold a maximum of two electrons of opposite spins. The maximum number of electrons in. Formula For Writing Electronic Configuration.
From classnotes.org.in
Electronic Configuration of Elements Structure of Atom Formula For Writing Electronic Configuration 3 rules of writing electronic configurations of atoms 1. By the end of this section, you will be able to: The electron configuration of an atom is written with the help of subshell labels. In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. Electronic configuration or. Formula For Writing Electronic Configuration.
From www.coursehero.com
[Solved] Write the full electron configurations for the following Formula For Writing Electronic Configuration Each orbital can hold a maximum of two electrons of opposite spins. By the end of this section, you will be able to: What is the electron configuration and orbital diagram of: In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. First, write out the electron.. Formula For Writing Electronic Configuration.
From www.youtube.com
Simple trick to write the electronic configuration easily YouTube Formula For Writing Electronic Configuration By the end of this section, you will be able to: The electron configuration of an atom is written with the help of subshell labels. The maximum number of electrons in the main quantum. Electronic configuration or electron configuration of orbitals takes place according to the following rules. The first integer, 1, gives us the principle energy level, the letter. Formula For Writing Electronic Configuration.