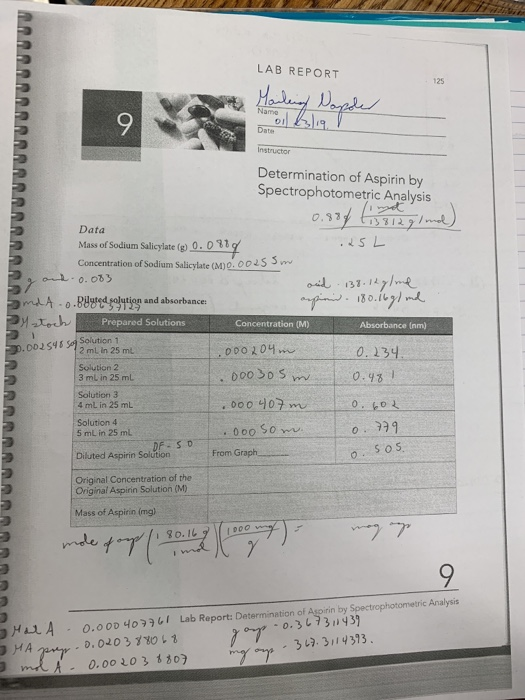
Spectrophotometric Analysis Of Aspirin Lab Answers . The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: Spectrophotometric analysis of commercial aspirin by: To learn about the absorption of light by. A colored complex is formed between aspirin and the iron (iii) ion. Experiment 613 spectrophotometric determination of aspirin section 1: The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. Purpose and summary most aspirin tablets are said to contain 5 grains of.
from lemurianembassy.com
In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. To learn about the absorption of light by. Experiment 613 spectrophotometric determination of aspirin section 1: The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): A colored complex is formed between aspirin and the iron (iii) ion. Spectrophotometric analysis of commercial aspirin by: Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. Purpose and summary most aspirin tablets are said to contain 5 grains of.
😀 Determination of aspirin by spectrophotometric analysis lab report
Spectrophotometric Analysis Of Aspirin Lab Answers Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: Spectrophotometric analysis of commercial aspirin by: In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. Experiment 613 spectrophotometric determination of aspirin section 1: The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. To learn about the absorption of light by. The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. Purpose and summary most aspirin tablets are said to contain 5 grains of. Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: A colored complex is formed between aspirin and the iron (iii) ion.
From www.studocu.com
Spectrophotometric Analysis of Aspirin SPECTROPHOTOMETRIC ANALYSIS OF Spectrophotometric Analysis Of Aspirin Lab Answers To learn about the absorption of light by. The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. Experiment 613 spectrophotometric determination of aspirin section 1: A colored complex is formed between aspirin and the iron (iii) ion. Purpose and summary most aspirin tablets are said to contain 5 grains of. Spectrophotometric analysis of. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Solved Spectrophotometric Analysis of a Commercial Aspirin Spectrophotometric Analysis Of Aspirin Lab Answers The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. To learn about the absorption of light by. In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. A colored complex is formed between aspirin and the iron (iii) ion. Hayden casassa and jonathan baugh section. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Solved Spectrophotometric Analysis of Commercial Aspirin I Spectrophotometric Analysis Of Aspirin Lab Answers Spectrophotometric analysis of commercial aspirin by: Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. To learn about the absorption of light by. The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): Experiment. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.studocu.com
Experiment 02 Spectrophotometric Analysis of Commercial Aspirin Spectrophotometric Analysis Of Aspirin Lab Answers The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. The uv (ultraviolet) spectrophotometer will show. Spectrophotometric Analysis Of Aspirin Lab Answers.
From studylib.net
Spectrophotometric Analysis of Aspirin Spectrophotometric Analysis Of Aspirin Lab Answers Spectrophotometric analysis of commercial aspirin by: To learn about the absorption of light by. A colored complex is formed between aspirin and the iron (iii) ion. Experiment 613 spectrophotometric determination of aspirin section 1: The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. In this experiment, spectrophotometric method was. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Solved Refer To Exp 10 "Spectrophotometric Analysis Of As... Spectrophotometric Analysis Of Aspirin Lab Answers A colored complex is formed between aspirin and the iron (iii) ion. The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. To learn about the absorption of light by. The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): The purpose of. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Solved Spectrophotometric Determination of Aspirin Report Spectrophotometric Analysis Of Aspirin Lab Answers To learn about the absorption of light by. Experiment 613 spectrophotometric determination of aspirin section 1: The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. The safety precautions associated with working. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.studocu.com
Exp 11 Spectrophotometric Analysis of a Commercial Aspirin Tablet Spectrophotometric Analysis Of Aspirin Lab Answers Experiment 613 spectrophotometric determination of aspirin section 1: Spectrophotometric analysis of commercial aspirin by: The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. The uv (ultraviolet) spectrophotometer will show the absorbance. Spectrophotometric Analysis Of Aspirin Lab Answers.
From pubs.acs.org
Spectrophotometric Determination of Aspirin, Phenacetin, and Caffeine Spectrophotometric Analysis Of Aspirin Lab Answers The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. To learn about the absorption of light by. Spectrophotometric analysis of commercial aspirin by: The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.studocu.com
Exp 12 Spectrophotometric Analysis Aspirin Tablet NEW EXPERIMENT 12 Spectrophotometric Analysis Of Aspirin Lab Answers Spectrophotometric analysis of commercial aspirin by: The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: To learn about the absorption of light by. The purpose. Spectrophotometric Analysis Of Aspirin Lab Answers.
From studylib.net
spectrophotometric analysis of aspirin Spectrophotometric Analysis Of Aspirin Lab Answers The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. A colored complex is formed between aspirin and. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Solved Spectrophotometric Analysis of Commercial Aspirin I Spectrophotometric Analysis Of Aspirin Lab Answers Experiment 613 spectrophotometric determination of aspirin section 1: Purpose and summary most aspirin tablets are said to contain 5 grains of. Spectrophotometric analysis of commercial aspirin by: The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. A colored complex is formed between aspirin and the iron (iii) ion. To learn about the absorption. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.studocu.com
Experiment 5 Report Spectrophotometric Analysis of Aspirin Studocu Spectrophotometric Analysis Of Aspirin Lab Answers The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. Purpose and summary most aspirin tablets are said to contain 5 grains of. The purpose of this experiment was to determine the percentage of aspirin in. Spectrophotometric Analysis Of Aspirin Lab Answers.
From lemurianembassy.com
😀 Determination of aspirin by spectrophotometric analysis lab report Spectrophotometric Analysis Of Aspirin Lab Answers The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: To learn about the absorption of light by. In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. A colored complex is formed. Spectrophotometric Analysis Of Aspirin Lab Answers.
From slideplayer.com
Spectrophotometric Analysis of Aspirin ppt video online download Spectrophotometric Analysis Of Aspirin Lab Answers The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. Experiment 613 spectrophotometric determination of aspirin section 1: Purpose and summary most. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Spectrophotometric Analysis of Aspirin Lab G Report Spectrophotometric Analysis Of Aspirin Lab Answers To learn about the absorption of light by. The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): Experiment 613 spectrophotometric determination of aspirin section 1: In this experiment, spectrophotometric method was applied. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Solved Spectrophotometric Analysis of Commercial Aspirin I Spectrophotometric Analysis Of Aspirin Lab Answers Spectrophotometric analysis of commercial aspirin by: The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. A colored complex is formed between aspirin and the iron (iii) ion. In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. The safety precautions associated with working with sodium. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.studocu.com
Experiment 3 THE Spectrophotometric Analysis OF Aspirin Page 1 Spectrophotometric Analysis Of Aspirin Lab Answers The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): Purpose and summary most aspirin tablets are said to contain 5 grains of. The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. The purpose of this experiment is to evaluate the percent. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Spectrophotometric Analysis of Aspirin Lab Report Spectrophotometric Analysis Of Aspirin Lab Answers The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. To learn about the absorption of light by. Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: Experiment 613 spectrophotometric determination of aspirin section 1: Purpose and summary most aspirin tablets are said to contain 5 grains of. Spectrophotometric analysis of. Spectrophotometric Analysis Of Aspirin Lab Answers.
From lemurianembassy.com
😀 Determination of aspirin by spectrophotometric analysis lab report Spectrophotometric Analysis Of Aspirin Lab Answers A colored complex is formed between aspirin and the iron (iii) ion. To learn about the absorption of light by. Experiment 613 spectrophotometric determination of aspirin section 1: In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: Spectrophotometric analysis. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Spectrophotometric Analysis of Aspirin Lab G Report Spectrophotometric Analysis Of Aspirin Lab Answers To learn about the absorption of light by. Spectrophotometric analysis of commercial aspirin by: A colored complex is formed between aspirin and the iron (iii) ion. Purpose and summary most aspirin tablets are said to contain 5 grains of. The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. Hayden casassa and jonathan baugh. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.youtube.com
Spectrophotometric Analysis of Aspirin inar 2022 10 26 YouTube Spectrophotometric Analysis Of Aspirin Lab Answers The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): Experiment 613 spectrophotometric determination of aspirin section 1: The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. Purpose and summary most aspirin tablets are said to contain 5 grains of. In this experiment, spectrophotometric method was. Spectrophotometric Analysis Of Aspirin Lab Answers.
From lemurianembassy.com
😀 Determination of aspirin by spectrophotometric analysis lab report Spectrophotometric Analysis Of Aspirin Lab Answers To learn about the absorption of light by. Spectrophotometric analysis of commercial aspirin by: The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.academia.edu
(DOC) Spectrophotometric Analysis of Aspirin Sidra Fatima Academia.edu Spectrophotometric Analysis Of Aspirin Lab Answers To learn about the absorption of light by. The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): Spectrophotometric analysis of commercial aspirin by: The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. The purpose of this experiment is to evaluate the percent aspirin on a. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.studocu.com
EXPERIMENT 5 SPECTROPHOTOMETRIC ANALYSIS OF A COMMERCIAL ASPIRIN TABLET Spectrophotometric Analysis Of Aspirin Lab Answers To learn about the absorption of light by. Spectrophotometric analysis of commercial aspirin by: The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. A colored complex is formed between aspirin and the iron (iii) ion. The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): Hayden. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Solved Determination of Aspirin by Spectrophotometric Spectrophotometric Analysis Of Aspirin Lab Answers Experiment 613 spectrophotometric determination of aspirin section 1: The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): A colored complex is formed between aspirin and the iron (iii) ion. The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. Hayden casassa and jonathan baugh section 0903. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Exp. 9 Spectrophotometric Determination of Aspirin Spectrophotometric Analysis Of Aspirin Lab Answers Experiment 613 spectrophotometric determination of aspirin section 1: The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. A colored complex is formed between aspirin and the iron (iii) ion. In this experiment,. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.chegg.com
Solved SPECTROPHOTOMETRIC ANALYSIS OF ASPIRIN 1. 0.3942g Spectrophotometric Analysis Of Aspirin Lab Answers The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): Purpose and summary most aspirin tablets are said to contain 5 grains of. Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: Experiment 613. Spectrophotometric Analysis Of Aspirin Lab Answers.
From studylib.net
1—Spectrophotometric Analysis of Commercial Aspirin Spectrophotometric Analysis Of Aspirin Lab Answers Purpose and summary most aspirin tablets are said to contain 5 grains of. The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. Experiment 613 spectrophotometric determination of aspirin section 1: Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: The safety precautions associated with working with sodium hydroxide in a. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.studocu.com
Prac 5 Spectrophotometric determination of aspirin in a commercial Spectrophotometric Analysis Of Aspirin Lab Answers Spectrophotometric analysis of commercial aspirin by: The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. A colored complex is formed between aspirin and the iron (iii) ion. In this experiment, spectrophotometric method was. Spectrophotometric Analysis Of Aspirin Lab Answers.
From www.numerade.com
SOLVED Lab 8 Spectrophotometric Analysis of Aspirin Introduction Spectrophotometric Analysis Of Aspirin Lab Answers Purpose and summary most aspirin tablets are said to contain 5 grains of. The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. The safety precautions associated with working with sodium hydroxide in a laboratory include (check all that apply): To learn about the absorption of light by. Experiment 613. Spectrophotometric Analysis Of Aspirin Lab Answers.
From studylib.net
Spectrophotometric Analysis of Aspirin Spectrophotometric Analysis Of Aspirin Lab Answers The uv (ultraviolet) spectrophotometer will show the absorbance due to sodium salicylate (from aspirin) present in. Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: Purpose and summary most aspirin tablets are said to contain 5 grains of. To learn about the absorption of light by. Spectrophotometric analysis of commercial aspirin by: The safety precautions associated with. Spectrophotometric Analysis Of Aspirin Lab Answers.
From studylib.net
Spectrophotometric Analysis of Aspirin Standards Data Spectrophotometric Analysis Of Aspirin Lab Answers A colored complex is formed between aspirin and the iron (iii) ion. The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. Purpose and summary most aspirin tablets are said to contain 5 grains. Spectrophotometric Analysis Of Aspirin Lab Answers.
From studylib.net
analysis of aspirin spectrophotometry Spectrophotometric Analysis Of Aspirin Lab Answers In this experiment, spectrophotometric method was applied to analyze aspirin in commercial aspirin product because this method is. Spectrophotometric analysis of commercial aspirin by: Hayden casassa and jonathan baugh section 0903 february 7th, 2016 learning objectives: The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. A colored complex is. Spectrophotometric Analysis Of Aspirin Lab Answers.
From studylib.net
Spectrophotometric Analysis of Aspirin Spectrophotometric Analysis Of Aspirin Lab Answers The purpose of this experiment was to determine the percentage of aspirin in an unknown sample through the use of. Purpose and summary most aspirin tablets are said to contain 5 grains of. The purpose of this experiment is to evaluate the percent aspirin on a commercial aspirin tablet. In this experiment, spectrophotometric method was applied to analyze aspirin in. Spectrophotometric Analysis Of Aspirin Lab Answers.