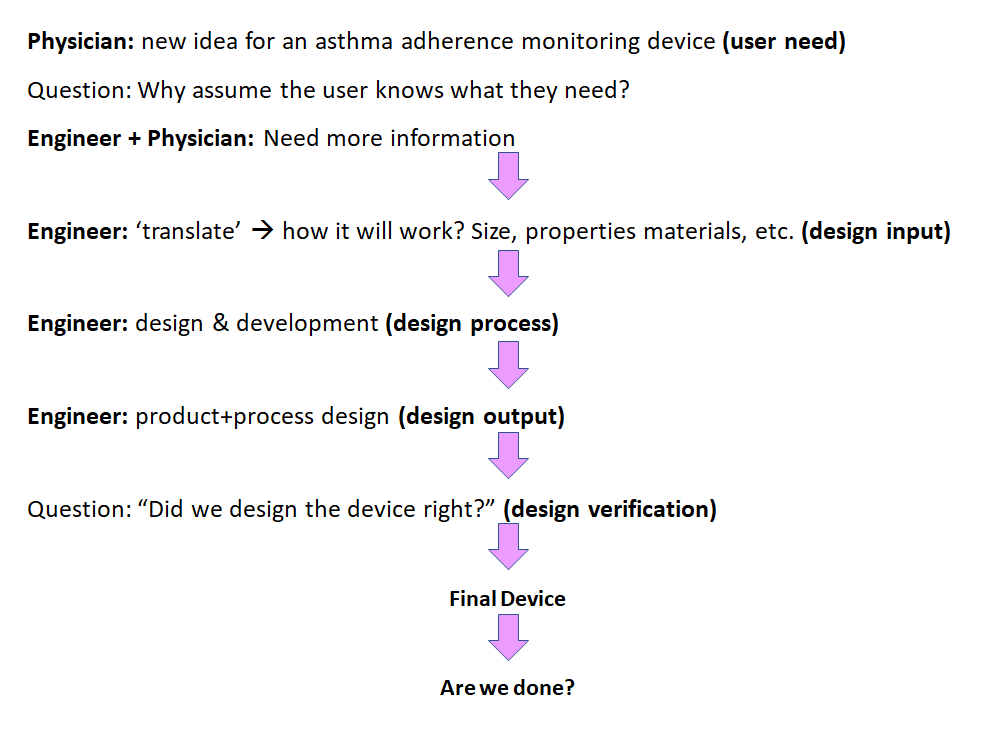
Medical Device Design Validation Example . To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. Design verification and design validation. In medical device development, two critical stages of the design process are often confused: As part of design and development validation, the organization shall perform clinical evaluations or performance. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Unravel the distinction between design verification and design validation in the realm of medical devices. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. What are their distinct roles in the delivery of quality. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be.
from www.kolabtree.com
According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. In medical device development, two critical stages of the design process are often confused: Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. What are their distinct roles in the delivery of quality. To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. Design verification and design validation. As part of design and development validation, the organization shall perform clinical evaluations or performance. Unravel the distinction between design verification and design validation in the realm of medical devices. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation.
Medical Device Design The Essential, StepbyStep Guide
Medical Device Design Validation Example If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. Design verification and design validation. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. What are their distinct roles in the delivery of quality. In medical device development, two critical stages of the design process are often confused: According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Unravel the distinction between design verification and design validation in the realm of medical devices. As part of design and development validation, the organization shall perform clinical evaluations or performance.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Design Validation Example If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. What. Medical Device Design Validation Example.
From www.aplyon.com
Design Verification and Validation Procedure Medical Device Design Validation Example As part of design and development validation, the organization shall perform clinical evaluations or performance. Unravel the distinction between design verification and design validation in the realm of medical devices. What are their distinct roles in the delivery of quality. To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have.. Medical Device Design Validation Example.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Medical Device Design Validation Example To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. As. Medical Device Design Validation Example.
From data1.skinnyms.com
Medical Device Verification And Validation Plan Template Medical Device Design Validation Example Design verification and design validation. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. In medical device development, two critical stages of the design process are often confused: If. Medical Device Design Validation Example.
From www.youtube.com
Developing a Testing Plan for Medical Device Design Verification YouTube Medical Device Design Validation Example In medical device development, two critical stages of the design process are often confused: As part of design and development validation, the organization shall perform clinical evaluations or performance. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. If you develop products — medical devices, particularly — then you’ve. Medical Device Design Validation Example.
From template.mapadapalavra.ba.gov.br
Medical Device Design And Development Plan Template Medical Device Design Validation Example To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. What are their distinct roles in the delivery of quality. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Unravel the distinction between design verification and design validation in. Medical Device Design Validation Example.
From www.greenlight.guru
The Beginner's Guide to Design Verification and Design Validation for Medical Device Design Validation Example Unravel the distinction between design verification and design validation in the realm of medical devices. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. As part of design and development validation, the organization shall perform clinical evaluations or performance. Safe medical device act of 1990 authorized fda to add design. Medical Device Design Validation Example.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Design Validation Example If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Design verification and design validation. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. What are their distinct roles in the delivery of quality. According to guidance published by the. Medical Device Design Validation Example.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Medical Device Design Validation Example What are their distinct roles in the delivery of quality. Unravel the distinction between design verification and design validation in the realm of medical devices. As part of design and development validation, the organization shall perform clinical evaluations or performance. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements.. Medical Device Design Validation Example.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Design Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. As. Medical Device Design Validation Example.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Medical Device Design Validation Example Design verification and design validation. What are their distinct roles in the delivery of quality. In medical device development, two critical stages of the design process are often confused: According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. To demonstrate that the manufactured medical device meets the design specifications,. Medical Device Design Validation Example.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Medical Device Design Validation Example Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. What are their distinct roles in the delivery of quality. Design verification and design validation. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. In medical device development, two. Medical Device Design Validation Example.
From www.scribd.com
Medical Device Design Verification SOP Medical Device Design Validation Example What are their distinct roles in the delivery of quality. Design verification and design validation. As part of design and development validation, the organization shall perform clinical evaluations or performance. In medical device development, two critical stages of the design process are often confused: If you develop products — medical devices, particularly — then you’ve heard the terms and design. Medical Device Design Validation Example.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Design Validation Example In medical device development, two critical stages of the design process are often confused: According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. What are their distinct roles in. Medical Device Design Validation Example.
From www.cognidox.com
Design verification & design validation for medical device developers Medical Device Design Validation Example As part of design and development validation, the organization shall perform clinical evaluations or performance. In medical device development, two critical stages of the design process are often confused: Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Design verification and design validation. According to guidance published by the. Medical Device Design Validation Example.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device Design Validation Example Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Unravel. Medical Device Design Validation Example.
From flamlabelthema.netlify.app
Medical Device Design Validation Protocol Template Medical Device Design Validation Example If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. As part of design and development validation, the organization shall perform clinical evaluations or performance. What are their distinct roles in the delivery of quality. According to guidance published by the ghtf (now the imdrf), the following are common examples of. Medical Device Design Validation Example.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Device Design Validation Example What are their distinct roles in the delivery of quality. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. To demonstrate that the manufactured medical device meets the design. Medical Device Design Validation Example.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Design Validation Example Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. What are their distinct roles in the delivery of quality. In medical device development, two critical stages of the design process are often confused: To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof. Medical Device Design Validation Example.
From www.slideteam.net
Medical Device Process Validation Flowchart Medical Device Design Validation Example Unravel the distinction between design verification and design validation in the realm of medical devices. Design verification and design validation. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have.. Medical Device Design Validation Example.
From www.emergobyul.com
Analysis of design validation and verification for medical device human Medical Device Design Validation Example Unravel the distinction between design verification and design validation in the realm of medical devices. In medical device development, two critical stages of the design process are often confused: If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. As part of design and development validation, the organization shall perform clinical. Medical Device Design Validation Example.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Design Validation Example To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. Design verification and design validation. In medical device development, two critical stages of the design process are often confused: What are their distinct roles in the delivery of quality. Safe medical device act of 1990 authorized fda to add design. Medical Device Design Validation Example.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Medical Device Design Validation Example If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Unravel the distinction between design verification and design validation in the realm of medical devices. To demonstrate that the manufactured medical. Medical Device Design Validation Example.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Design Validation Example In medical device development, two critical stages of the design process are often confused: What are their distinct roles in the delivery of quality. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Unravel the distinction between design verification and design validation in the realm of medical devices. If. Medical Device Design Validation Example.
From studylib.net
Medical Device Product Verification and Validation Medical Device Design Validation Example In medical device development, two critical stages of the design process are often confused: Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Design verification and design validation. What are. Medical Device Design Validation Example.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Medical Device Design Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. What. Medical Device Design Validation Example.
From qaconsultinginc.com
Design Verification vs. Design Validation What’s The Difference? QA Medical Device Design Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. As part of design and development validation, the organization shall perform clinical evaluations or performance. In medical device development, two critical stages of the design process are often confused: What are their distinct roles in the delivery of quality. Unravel. Medical Device Design Validation Example.
From www.scribd.com
Medical Device Design Validation SOP Verification And Validation Medical Device Design Validation Example As part of design and development validation, the organization shall perform clinical evaluations or performance. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Unravel the distinction between design verification and design validation in the realm of medical devices. According to guidance published by the ghtf (now the imdrf), the. Medical Device Design Validation Example.
From rs-ness.com
Medical Device Packaging Validation Guideline RS NESS Medical Device Design Validation Example Unravel the distinction between design verification and design validation in the realm of medical devices. As part of design and development validation, the organization shall perform clinical evaluations or performance. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Design verification and design validation. If you develop products —. Medical Device Design Validation Example.
From medicaldevicehq.com
Medical device design control terminology Medical Device HQ 1 Medical Device Design Validation Example As part of design and development validation, the organization shall perform clinical evaluations or performance. To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. In medical device development, two critical stages of the design process are often confused: Unravel the distinction between design verification and design validation in the. Medical Device Design Validation Example.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Design Validation Example Design verification and design validation. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. What are their distinct roles in the delivery of quality. Unravel the distinction between design. Medical Device Design Validation Example.
From www.perforce.com
Design Verification/Validation for Med Device Development Perforce Medical Device Design Validation Example In medical device development, two critical stages of the design process are often confused: According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Design verification and design validation. To demonstrate that the manufactured medical device meets the design specifications, there must be documented proof that the ctqs have. Unravel. Medical Device Design Validation Example.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Medical Device Design Validation Example If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Design verification and design validation. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. In medical device development, two critical stages of the design process are often confused: According to. Medical Device Design Validation Example.
From www.proplate.com
Medical Device Design & Development Electroplating Solutions ProPlate Medical Device Design Validation Example In medical device development, two critical stages of the design process are often confused: What are their distinct roles in the delivery of quality. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Design verification and design validation. Unravel the distinction between design verification and design validation in the. Medical Device Design Validation Example.
From www.qualitymeddev.com
Design Verification vs Design Validation What are The Differences Medical Device Design Validation Example What are their distinct roles in the delivery of quality. Unravel the distinction between design verification and design validation in the realm of medical devices. In medical device development, two critical stages of the design process are often confused: As part of design and development validation, the organization shall perform clinical evaluations or performance. Design verification and design validation. According. Medical Device Design Validation Example.