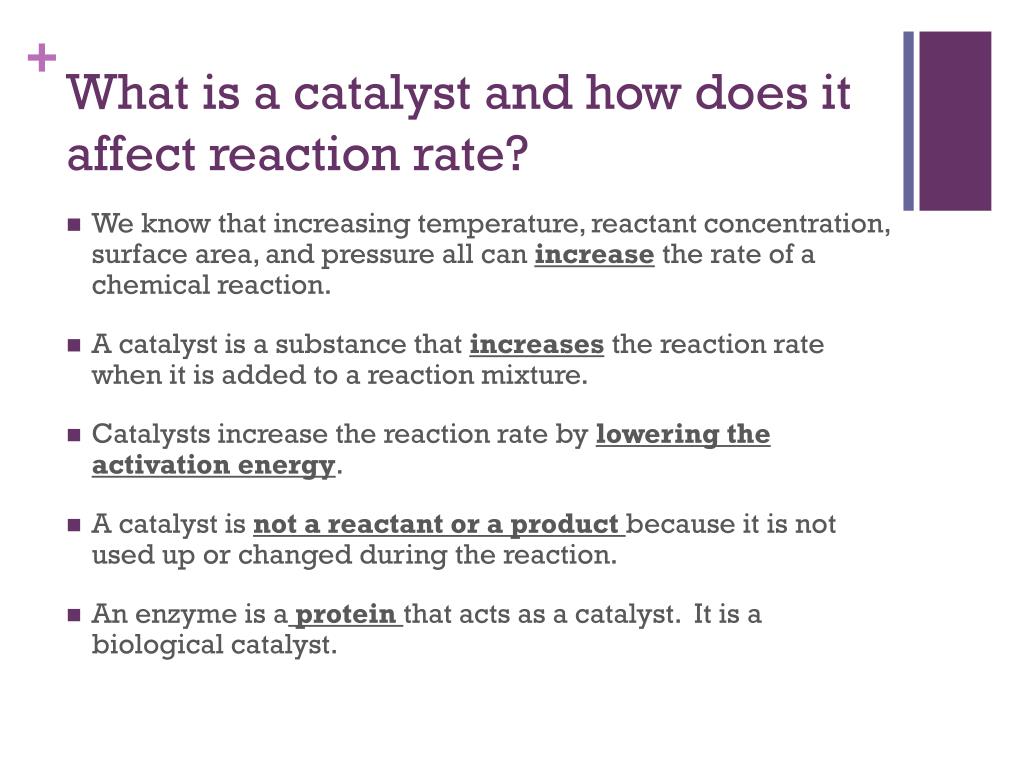
A Catalyst Increase The Rate Of A Reaction By . only a very small mass of catalyst is needed to increase the rate of a reaction. Of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. They do this by changing the reaction pathway. It assumes that you are already familiar with basic ideas about the. catalysts increase the rate of chemical reactions by lowering the activation energy. mass is measured in kilograms (kg) or grams (g). this page describes and explains the way that adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. this page explains how adding a catalyst affects the rate of a reaction.
from www.slideserve.com
However, not all reactions have suitable catalysts. They do this by changing the reaction pathway. this page describes and explains the way that adding a catalyst affects the rate of a reaction. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. mass is measured in kilograms (kg) or grams (g). catalysts increase the rate of chemical reactions by lowering the activation energy. only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic ideas about the. Of catalyst is needed to increase the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface.
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687
A Catalyst Increase The Rate Of A Reaction By However, not all reactions have suitable catalysts. They do this by changing the reaction pathway. However, not all reactions have suitable catalysts. this page describes and explains the way that adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. this page explains how adding a catalyst affects the rate of a reaction. catalysts increase the rate of chemical reactions by lowering the activation energy. only a very small mass of catalyst is needed to increase the rate of a reaction. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. It assumes that you are already familiar with basic ideas about the. Of catalyst is needed to increase the rate of a reaction. mass is measured in kilograms (kg) or grams (g).
From exohgcpop.blob.core.windows.net
A Catalyst Increases The Rate Of Reaction As It at Gladys McCoy blog A Catalyst Increase The Rate Of A Reaction By mass is measured in kilograms (kg) or grams (g). this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts increase the rate of chemical reactions by lowering the activation energy. Of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. It. A Catalyst Increase The Rate Of A Reaction By.
From slideplayer.com
Reaction Rates and Chemical Equilibrium ppt download A Catalyst Increase The Rate Of A Reaction By However, not all reactions have suitable catalysts. Of catalyst is needed to increase the rate of a reaction. They do this by changing the reaction pathway. mass is measured in kilograms (kg) or grams (g). this page describes and explains the way that adding a catalyst affects the rate of a reaction. this page explains how adding. A Catalyst Increase The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Catalyst Increase The Rate Of A Reaction By this page describes and explains the way that adding a catalyst affects the rate of a reaction. this page explains how adding a catalyst affects the rate of a reaction. mass is measured in kilograms (kg) or grams (g). Of catalyst is needed to increase the rate of a reaction. only a very small mass of. A Catalyst Increase The Rate Of A Reaction By.
From slidetodoc.com
Energy Changes Reaction Rates and Equilibrium Thermodynamics study A Catalyst Increase The Rate Of A Reaction By this page explains how adding a catalyst affects the rate of a reaction. mass is measured in kilograms (kg) or grams (g). It assumes that you are already familiar with basic ideas about the. They do this by changing the reaction pathway. catalysts increase the rate of chemical reactions by lowering the activation energy. only a. A Catalyst Increase The Rate Of A Reaction By.
From www.youtube.com
How do enzymatic catalysts increase the rates of reactions? YouTube A Catalyst Increase The Rate Of A Reaction By this page describes and explains the way that adding a catalyst affects the rate of a reaction. They do this by changing the reaction pathway. mass is measured in kilograms (kg) or grams (g). It assumes that you are already familiar with basic ideas about the. catalysts increase the rate of chemical reactions by lowering the activation. A Catalyst Increase The Rate Of A Reaction By.
From askfilo.com
How does a catalyst increase the rate of a reaction 3 Filo A Catalyst Increase The Rate Of A Reaction By Of catalyst is needed to increase the rate of a reaction. only a very small mass of catalyst is needed to increase the rate of a reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. mass is measured in kilograms (kg) or grams (g). However, not all reactions. A Catalyst Increase The Rate Of A Reaction By.
From www.numerade.com
SOLVED Draw a reaction coordinate diagram for the reversible reaction A Catalyst Increase The Rate Of A Reaction By However, not all reactions have suitable catalysts. this page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the. Of catalyst is needed to increase the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing. A Catalyst Increase The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Catalyst Increase The Rate Of A Reaction By mass is measured in kilograms (kg) or grams (g). Of catalyst is needed to increase the rate of a reaction. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. They do this by changing the reaction pathway. this page explains how adding a. A Catalyst Increase The Rate Of A Reaction By.
From learnaboutchemistry.com
Can a Catalyst increase the rate of a reaction? » Learn About Chemistry A Catalyst Increase The Rate Of A Reaction By mass is measured in kilograms (kg) or grams (g). They do this by changing the reaction pathway. It assumes that you are already familiar with basic ideas about the. this page explains how adding a catalyst affects the rate of a reaction. Of catalyst is needed to increase the rate of a reaction. catalysts increase the rate. A Catalyst Increase The Rate Of A Reaction By.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction A Catalyst Increase The Rate Of A Reaction By Of catalyst is needed to increase the rate of a reaction. catalysts increase the rate of chemical reactions by lowering the activation energy. this page explains how adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. . A Catalyst Increase The Rate Of A Reaction By.
From www.chegg.com
Solved Catalysts increase reaction rates by A) increasing A Catalyst Increase The Rate Of A Reaction By only a very small mass of catalyst is needed to increase the rate of a reaction. catalysts increase the rate of chemical reactions by lowering the activation energy. It assumes that you are already familiar with basic ideas about the. Of catalyst is needed to increase the rate of a reaction. mass is measured in kilograms (kg). A Catalyst Increase The Rate Of A Reaction By.
From byjus.com
How does a catalyst increase the rate of a reaction? A Catalyst Increase The Rate Of A Reaction By this page explains how adding a catalyst affects the rate of a reaction. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. However, not all reactions have suitable catalysts. Of catalyst is needed to increase the rate of a reaction. catalysts increase the. A Catalyst Increase The Rate Of A Reaction By.
From studyfullfarrior.z19.web.core.windows.net
Rates Of Reaction And Energy Changes A Catalyst Increase The Rate Of A Reaction By They do this by changing the reaction pathway. catalysts increase the rate of chemical reactions by lowering the activation energy. It assumes that you are already familiar with basic ideas about the. only a very small mass of catalyst is needed to increase the rate of a reaction. students watch a video and do a quick activity. A Catalyst Increase The Rate Of A Reaction By.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID A Catalyst Increase The Rate Of A Reaction By revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. Of catalyst is needed to increase the rate of a reaction. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. It assumes that you. A Catalyst Increase The Rate Of A Reaction By.
From www.sasadoctor.com
Rate of reaction chemistry igcse A Catalyst Increase The Rate Of A Reaction By catalysts increase the rate of chemical reactions by lowering the activation energy. this page describes and explains the way that adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. Of catalyst is needed to increase the rate. A Catalyst Increase The Rate Of A Reaction By.
From www.bartleby.com
Answered Catalysts increase reaction rates by A)… bartleby A Catalyst Increase The Rate Of A Reaction By Of catalyst is needed to increase the rate of a reaction. only a very small mass of catalyst is needed to increase the rate of a reaction. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. They do this by changing the reaction pathway.. A Catalyst Increase The Rate Of A Reaction By.
From www.doubtnut.com
A catalyst increases rate of reaction by A Catalyst Increase The Rate Of A Reaction By this page explains how adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. only a very small mass of catalyst is needed to increase the rate of a reaction. Of catalyst is needed to increase the rate. A Catalyst Increase The Rate Of A Reaction By.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 A Catalyst Increase The Rate Of A Reaction By mass is measured in kilograms (kg) or grams (g). Of catalyst is needed to increase the rate of a reaction. only a very small mass of catalyst is needed to increase the rate of a reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts increase the. A Catalyst Increase The Rate Of A Reaction By.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at A Catalyst Increase The Rate Of A Reaction By revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. catalysts increase the rate of chemical reactions by lowering the activation energy. this. A Catalyst Increase The Rate Of A Reaction By.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 A Catalyst Increase The Rate Of A Reaction By They do this by changing the reaction pathway. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. mass is measured in kilograms (kg). A Catalyst Increase The Rate Of A Reaction By.
From www.numerade.com
SOLVEDDoes a catalyst increase reaction rate by the same means as a A Catalyst Increase The Rate Of A Reaction By However, not all reactions have suitable catalysts. catalysts increase the rate of chemical reactions by lowering the activation energy. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. students watch a video and do a quick activity to see that a catalyst can increase the rate. A Catalyst Increase The Rate Of A Reaction By.
From dxotzuihu.blob.core.windows.net
Why Does Catalyst Affect Reaction Rate at David Morfin blog A Catalyst Increase The Rate Of A Reaction By They do this by changing the reaction pathway. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. Of catalyst is needed to increase the rate of a reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. only. A Catalyst Increase The Rate Of A Reaction By.
From answerhappy.com
A catalyst is a substance that increases the rate of reaction but does A Catalyst Increase The Rate Of A Reaction By It assumes that you are already familiar with basic ideas about the. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. this page explains how adding a catalyst affects the rate of a reaction. mass is measured in kilograms (kg) or grams (g).. A Catalyst Increase The Rate Of A Reaction By.
From www.numerade.com
SOLVED Catalysts increase reaction rates by A) increasing the A Catalyst Increase The Rate Of A Reaction By revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. mass is measured in kilograms (kg) or grams (g). Of catalyst is needed to increase the rate of a reaction. this page explains how adding a catalyst affects the rate of a reaction. They do this by. A Catalyst Increase The Rate Of A Reaction By.
From solvedlib.com
How does a catalyst increase the rate of a chemical r… SolvedLib A Catalyst Increase The Rate Of A Reaction By catalysts increase the rate of chemical reactions by lowering the activation energy. this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the. Of catalyst is needed to increase the rate of a reaction. only a very small mass. A Catalyst Increase The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Catalyst Increase The Rate Of A Reaction By It assumes that you are already familiar with basic ideas about the. this page explains how adding a catalyst affects the rate of a reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. only a very small mass of catalyst is needed to increase the rate of a. A Catalyst Increase The Rate Of A Reaction By.
From www.numerade.com
SOLVEDA catalyst increases rate of reaction by (a) decreasing enthalpy A Catalyst Increase The Rate Of A Reaction By It assumes that you are already familiar with basic ideas about the. Of catalyst is needed to increase the rate of a reaction. mass is measured in kilograms (kg) or grams (g). only a very small mass of catalyst is needed to increase the rate of a reaction. this page explains how adding a catalyst affects the. A Catalyst Increase The Rate Of A Reaction By.
From chemistryguru.com.sg
Rate Concentration Graph for Enzyme Catalysed Reaction A Catalyst Increase The Rate Of A Reaction By this page describes and explains the way that adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. this page explains how adding a catalyst affects the rate of a reaction. catalysts increase the rate of chemical reactions by lowering the activation energy. revise how to measure rates of reaction. A Catalyst Increase The Rate Of A Reaction By.
From www.slideserve.com
PPT Chapter 5 Chemical Quantities and Reactions PowerPoint A Catalyst Increase The Rate Of A Reaction By They do this by changing the reaction pathway. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. Of catalyst is needed to increase the rate of a reaction. this page describes and explains the way that adding a catalyst affects the rate of a. A Catalyst Increase The Rate Of A Reaction By.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry A Catalyst Increase The Rate Of A Reaction By this page explains how adding a catalyst affects the rate of a reaction. They do this by changing the reaction pathway. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. only a very small mass of catalyst is needed to increase the rate of a reaction.. A Catalyst Increase The Rate Of A Reaction By.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions A Catalyst Increase The Rate Of A Reaction By students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. Of catalyst is needed to increase the rate of a reaction. mass is measured in kilograms (kg) or grams (g). It assumes that you are already familiar with basic ideas about the. this page. A Catalyst Increase The Rate Of A Reaction By.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 A Catalyst Increase The Rate Of A Reaction By this page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the. mass is measured in kilograms (kg) or grams (g). They do this by changing the reaction pathway. this page describes and explains the way that adding a catalyst affects the rate of. A Catalyst Increase The Rate Of A Reaction By.
From exohdpwxs.blob.core.windows.net
Catalyst Shapes at Roberto Davis blog A Catalyst Increase The Rate Of A Reaction By They do this by changing the reaction pathway. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. mass is measured in kilograms (kg). A Catalyst Increase The Rate Of A Reaction By.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube A Catalyst Increase The Rate Of A Reaction By Of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. this page explains how adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. catalysts increase the rate of chemical. A Catalyst Increase The Rate Of A Reaction By.
From helecu.com
Factors Affecting Reaction Rates Chemistry Atoms First (2022) A Catalyst Increase The Rate Of A Reaction By It assumes that you are already familiar with basic ideas about the. this page describes and explains the way that adding a catalyst affects the rate of a reaction. students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of. Of catalyst is needed to increase. A Catalyst Increase The Rate Of A Reaction By.