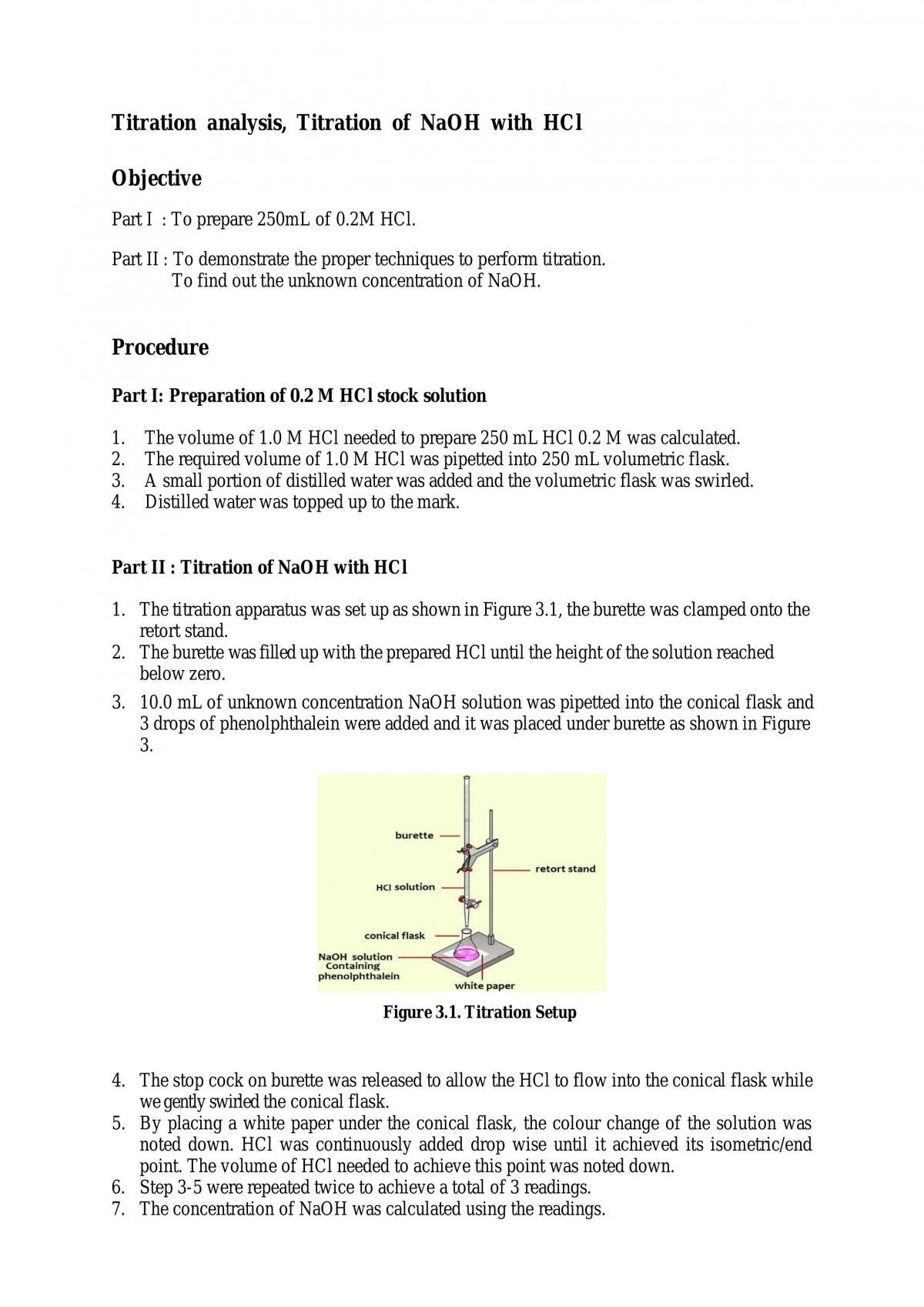
Titration Lab Calculations Naoh And Hcl . hcl + naoh → nacl + h 2 o. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. \ce{naoh}\) is required to reach the end. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. When the acid and base react,. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? The following examples work through these kinds of titration calculations: suppose that a titration is performed and \(20.70 \:
from www.vrogue.co
in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). The following examples work through these kinds of titration calculations: hcl + naoh → nacl + h 2 o. suppose that a titration is performed and \(20.70 \: When the acid and base react,. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. \ce{naoh}\) is required to reach the end. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute.
Solved Lab Report Acid Base Titration Using Acids Hcl vrogue.co
Titration Lab Calculations Naoh And Hcl suppose that a titration is performed and \(20.70 \: hcl + naoh → nacl + h 2 o. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? When the acid and base react,. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The following examples work through these kinds of titration calculations: titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide).
From mungfali.com
HCl NaOH Titration Titration Lab Calculations Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. suppose that a titration is performed and \(20.70 \: Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been. Titration Lab Calculations Naoh And Hcl.
From www.vrogue.co
Solved Lab Report Acid Base Titration Using Acids Hcl vrogue.co Titration Lab Calculations Naoh And Hcl in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. \ce{naoh}\) is required to reach the end. When the acid and base react,. titrations where the analyte is a weak base or acid will need. Titration Lab Calculations Naoh And Hcl.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Titration Lab Calculations Naoh And Hcl suppose that a titration is performed and \(20.70 \: if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. titrations where. Titration Lab Calculations Naoh And Hcl.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Titration Lab Calculations Naoh And Hcl use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. hcl + naoh → nacl + h 2 o. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: The following examples work through these kinds of titration calculations: if you know. Titration Lab Calculations Naoh And Hcl.
From www.studocu.com
Hcl naoh lab lab report Chem 1252, General Chemistry I Lab Johnson Titration Lab Calculations Naoh And Hcl in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. The following examples work through these kinds of titration calculations: in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). suppose that a titration is performed and \(20.70. Titration Lab Calculations Naoh And Hcl.
From www.studypool.com
SOLUTION Chemistry lab report about titration between naoh and hcl Titration Lab Calculations Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. When the acid and base react,. \ce{naoh}\) is required to reach the end. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm. Titration Lab Calculations Naoh And Hcl.
From www.chegg.com
Solved Experiment Estimation of sodiumhydroxide using Titration Lab Calculations Naoh And Hcl titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). The following examples work through these kinds of titration calculations: Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. . Titration Lab Calculations Naoh And Hcl.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Lab Calculations Naoh And Hcl \ce{naoh}\) is required to reach the end. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). suppose that a titration is performed and \(20.70 \: The following examples work through these kinds of titration calculations: When the acid and base react,. if you know that titrating 50.00 ml of an. Titration Lab Calculations Naoh And Hcl.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Titration Lab Calculations Naoh And Hcl use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. \ce{naoh}\) is required to reach the end. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised. Titration Lab Calculations Naoh And Hcl.
From dxowlwwmg.blob.core.windows.net
Titration Experiment Titration Results Table at Barbara Dorman blog Titration Lab Calculations Naoh And Hcl in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). use this class. Titration Lab Calculations Naoh And Hcl.
From www.youtube.com
Titration calculation 1 NaOH Vs HCl YouTube Titration Lab Calculations Naoh And Hcl use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. titrations where the analyte is a. Titration Lab Calculations Naoh And Hcl.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration Lab Calculations Naoh And Hcl The following examples work through these kinds of titration calculations: hcl + naoh → nacl + h 2 o. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). . Titration Lab Calculations Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Titration Lab Calculations Naoh And Hcl in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). use this class. Titration Lab Calculations Naoh And Hcl.
From www.numerade.com
SOLVED The following data were collected during a titration Calculate Titration Lab Calculations Naoh And Hcl if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of. Titration Lab Calculations Naoh And Hcl.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Titration Lab Calculations Naoh And Hcl in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \:. Titration Lab Calculations Naoh And Hcl.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Lab Calculations Naoh And Hcl The following examples work through these kinds of titration calculations: Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. suppose that a titration is performed and \(20.70 \: Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? in a titration, 25.00. Titration Lab Calculations Naoh And Hcl.
From exoqledql.blob.core.windows.net
Titration Base Hydrochloric Acid at Bryan Barnes blog Titration Lab Calculations Naoh And Hcl When the acid and base react,. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. The following examples work through these kinds of titration calculations: use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide. Titration Lab Calculations Naoh And Hcl.
From www.numerade.com
SOLVED 4) (4 pts) In lab you titrated HCl with NaOH Sketch the Titration Lab Calculations Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except. Titration Lab Calculations Naoh And Hcl.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titration Lab Calculations Naoh And Hcl \ce{naoh}\) is required to reach the end. hcl + naoh → nacl + h 2 o. When the acid and base react,. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Example \(\pageindex{3}\) what is the ph when 48.00 ml. Titration Lab Calculations Naoh And Hcl.
From dxopcuxtp.blob.core.windows.net
Titration Experiment Hydrochloric Acid And Sodium Hydroxide at Silvia Titration Lab Calculations Naoh And Hcl titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). suppose that a titration is performed and \(20.70 \: The following examples work through these kinds of titration calculations: \ce{naoh}\). Titration Lab Calculations Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Titration Lab Calculations Naoh And Hcl in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). When the acid and base react,. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. suppose that a titration is performed and \(20.70. Titration Lab Calculations Naoh And Hcl.
From www.studypool.com
SOLUTION Rvs chemistry 11 hydrochloric acid and sodium hydroxide Titration Lab Calculations Naoh And Hcl suppose that a titration is performed and \(20.70 \: The following examples work through these kinds of titration calculations: When the acid and base react,. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. titrations where the analyte is a weak base or acid will need equilibrium /. Titration Lab Calculations Naoh And Hcl.
From dxoinphla.blob.core.windows.net
Titration Hcl Vs Na2Co3 at Ahmad Panos blog Titration Lab Calculations Naoh And Hcl \ce{naoh}\) is required to reach the end. The following examples work through these kinds of titration calculations: hcl + naoh → nacl + h 2 o. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a. Titration Lab Calculations Naoh And Hcl.
From vdocuments.mx
Acid and Base Titrations MhChemPage I38 / Acid and Base Titrations Titration Lab Calculations Naoh And Hcl suppose that a titration is performed and \(20.70 \: in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? . Titration Lab Calculations Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Titration Lab Calculations Naoh And Hcl titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). hcl + naoh. Titration Lab Calculations Naoh And Hcl.
From exoqledql.blob.core.windows.net
Titration Base Hydrochloric Acid at Bryan Barnes blog Titration Lab Calculations Naoh And Hcl in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh,. Titration Lab Calculations Naoh And Hcl.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Titration Lab Calculations Naoh And Hcl use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The following examples work through these kinds of titration calculations: hcl + naoh → nacl + h 2 o. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can. Titration Lab Calculations Naoh And Hcl.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Titration Lab Calculations Naoh And Hcl if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? use this class practical to explore. Titration Lab Calculations Naoh And Hcl.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Titration Lab Calculations Naoh And Hcl in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. hcl + naoh → nacl + h. Titration Lab Calculations Naoh And Hcl.
From www.gulfsouthsolar.com
Extraordinario Solenoide Formación titration of sodium hydroxide with Titration Lab Calculations Naoh And Hcl When the acid and base react,. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. \ce{naoh}\) is required to reach the end. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Hydrochloric acid reacts with sodium hydroxide. Titration Lab Calculations Naoh And Hcl.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Titration Lab Calculations Naoh And Hcl in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). in a titration,. Titration Lab Calculations Naoh And Hcl.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Lab Calculations Naoh And Hcl titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). in a titration,. Titration Lab Calculations Naoh And Hcl.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Lab Calculations Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. The following examples work through these kinds of titration calculations: use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can. Titration Lab Calculations Naoh And Hcl.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration Lab Calculations Naoh And Hcl in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? The following examples work through these kinds of titration calculations: use this class practical to explore titration,. Titration Lab Calculations Naoh And Hcl.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Lab Calculations Naoh And Hcl in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. suppose that a titration is performed and \(20.70 \: When the acid and base react,. if you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you. Titration Lab Calculations Naoh And Hcl.