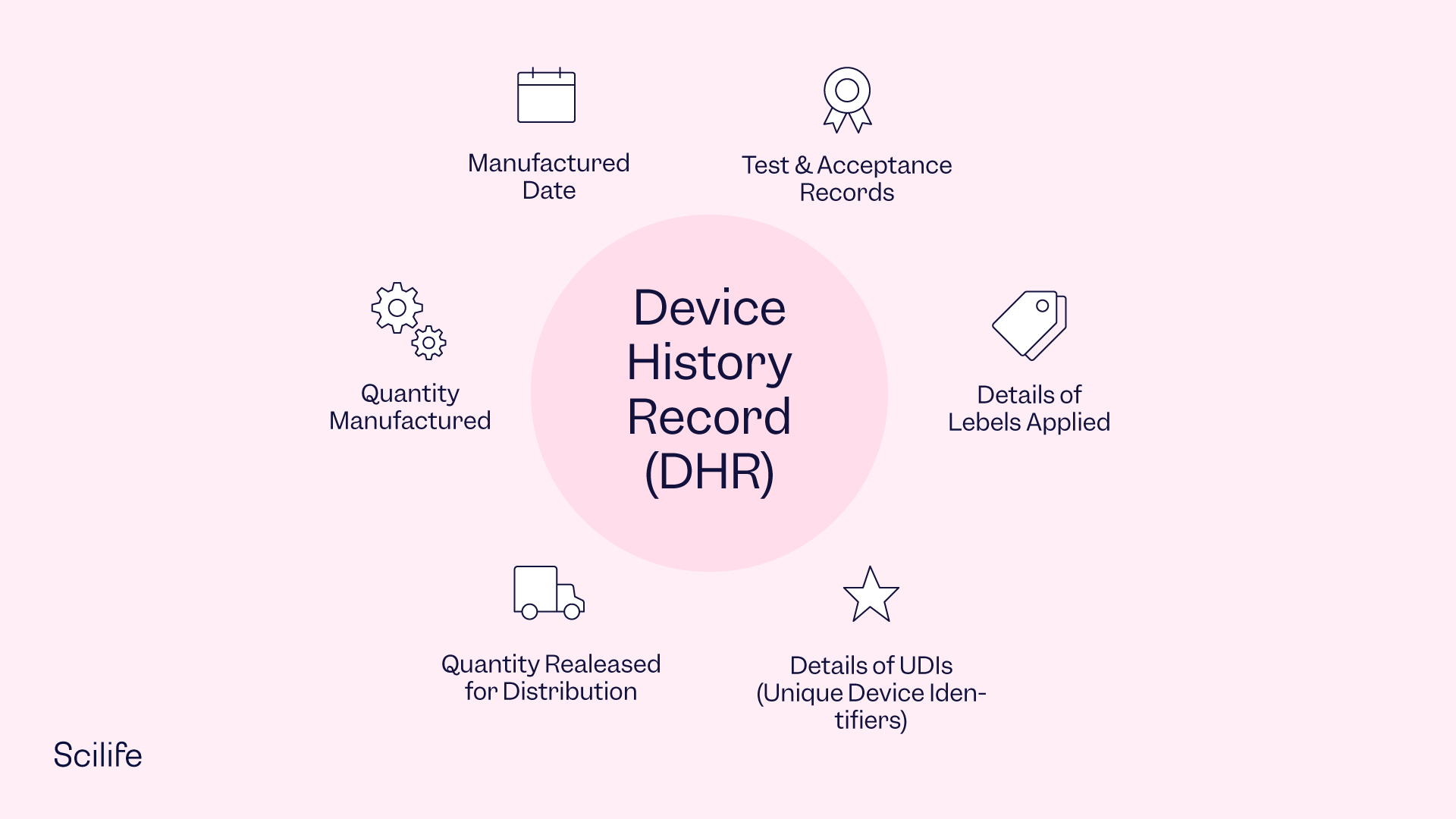
Device History Record Mdr . Each manufacturer shall establish and maintain procedures to ensure. Device history record (dhr) according to 21 cfr part 820.184; The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. C) comparison of the files. Each manufacturer shall maintain device history records (dhr's). What are the requirements for establishing and maintaining mdr files or records that apply to me? Device master record (dmr) according to 21 cfr part 820.181; The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of.
from www.scilife.io
Device history record (dhr) according to 21 cfr part 820.184; The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Device master record (dmr) according to 21 cfr part 820.181; C) comparison of the files. Each manufacturer shall establish and maintain procedures to ensure. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. Each manufacturer shall maintain device history records (dhr's). What are the requirements for establishing and maintaining mdr files or records that apply to me?
What is Device History Record (DHR)? Complete definition Scilife
Device History Record Mdr The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. Device master record (dmr) according to 21 cfr part 820.181; The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Device history record (dhr) according to 21 cfr part 820.184; Each manufacturer shall maintain device history records (dhr's). What are the requirements for establishing and maintaining mdr files or records that apply to me? Each manufacturer shall establish and maintain procedures to ensure. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. C) comparison of the files.
From aplyonqms.com
Device History Record Procedure A. P. LYON Device History Record Mdr The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. Each manufacturer shall establish and maintain procedures to ensure. Device history record (dhr) according to 21 cfr. Device History Record Mdr.
From hardcoreqms.com
Device Master Records (DMR) for Medical Devices (2023) Device History Record Mdr The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Each manufacturer shall maintain device history records (dhr's). The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Device master record (dmr) according to 21 cfr part 820.181; C) comparison of the files. Maintaining a complete. Device History Record Mdr.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Device History Record Mdr Each manufacturer shall establish and maintain procedures to ensure. C) comparison of the files. Device history record (dhr) according to 21 cfr part 820.184; The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device. Device History Record Mdr.
From www.scilife.io
What is Device History Record (DHR)? Complete definition Scilife Device History Record Mdr Each manufacturer shall establish and maintain procedures to ensure. C) comparison of the files. Device master record (dmr) according to 21 cfr part 820.181; Each manufacturer shall maintain device history records (dhr's). The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. The medical device reporting (mdr) regulation. Device History Record Mdr.
From www.youtube.com
Device History Record HD YouTube Device History Record Mdr The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Device master record (dmr) according to 21 cfr part 820.181; The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. Each manufacturer shall establish and maintain procedures to ensure. The medical. Device History Record Mdr.
From www.slideserve.com
PPT Design documentation PowerPoint Presentation, free download ID Device History Record Mdr C) comparison of the files. Device history record (dhr) according to 21 cfr part 820.184; Device master record (dmr) according to 21 cfr part 820.181; Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. The medical device reporting (mdr) regulation (21 cfr part. Device History Record Mdr.
From rs-ness.com
DHR is an essential requirement for Medical Device Company RS NESS Device History Record Mdr Each manufacturer shall establish and maintain procedures to ensure. The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. The medical device. Device History Record Mdr.
From www.instantgmp.com
Device History Record Device History Record Mdr The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Device history record (dhr) according to 21 cfr part 820.184; Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. The device history record (dhr), device master. Device History Record Mdr.
From www.youtube.com
Design History File, Device History Record, Device Master Record and Device History Record Mdr The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. Each manufacturer shall establish and maintain procedures to ensure. Maintaining a complete and accurate design history file (dhf) is mandatory for. Device History Record Mdr.
From slidetodoc.com
Design History File Device Master Record and Device Device History Record Mdr What are the requirements for establishing and maintaining mdr files or records that apply to me? Device master record (dmr) according to 21 cfr part 820.181; The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and.. Device History Record Mdr.
From templates.rjuuc.edu.np
Device History Record Template Device History Record Mdr C) comparison of the files. What are the requirements for establishing and maintaining mdr files or records that apply to me? Each manufacturer shall establish and maintain procedures to ensure. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Each manufacturer shall maintain device history records (dhr's). The device history record (dhr),. Device History Record Mdr.
From templates.rjuuc.edu.np
Device History Record Template Device History Record Mdr The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Device master record (dmr) according to 21 cfr part 820.181; Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. Each manufacturer shall establish and maintain procedures. Device History Record Mdr.
From www.youtube.com
Documentation Deconstructed Understanding the Technical file YouTube Device History Record Mdr C) comparison of the files. Device master record (dmr) according to 21 cfr part 820.181; Each manufacturer shall maintain device history records (dhr's). Each manufacturer shall establish and maintain procedures to ensure. Device history record (dhr) according to 21 cfr part 820.184; The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of. Device History Record Mdr.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device History Record Mdr Device history record (dhr) according to 21 cfr part 820.184; C) comparison of the files. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. Each manufacturer shall maintain device history records (dhr's). Each manufacturer shall establish and maintain procedures to ensure. Device master. Device History Record Mdr.
From aplyonqms.com
Device History Record Procedure A. P. LYON Device History Record Mdr The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. Each manufacturer shall establish and maintain procedures to ensure. Each manufacturer shall maintain device history records (dhr's). C) comparison of the files. Device master record (dmr) according to 21 cfr part 820.181; Device history record (dhr) according to. Device History Record Mdr.
From www.bizmanualz.com
Device Master Record Index Template Device History Record Mdr Each manufacturer shall establish and maintain procedures to ensure. The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. Each manufacturer shall maintain device history records (dhr's). Device master record (dmr) according to 21 cfr part 820.181; The medical device reporting (mdr) regulation (21 cfr part 803) contains. Device History Record Mdr.
From www.scilife.io
The 5 Medical Device Development Phases Scilife Device History Record Mdr What are the requirements for establishing and maintaining mdr files or records that apply to me? The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. C) comparison of the files. The device history record (dhr),. Device History Record Mdr.
From www.greenlight.guru
Medical Device Reporting (MDR) How to Take Advantage of Your Device History Record Mdr The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. Device master record (dmr) according to 21 cfr part 820.181; C) comparison of the files. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Maintaining a complete and accurate design. Device History Record Mdr.
From www.youtube.com
Design History File DHF, Device Master Record DMR, Device History Device History Record Mdr Device history record (dhr) according to 21 cfr part 820.184; Each manufacturer shall establish and maintain procedures to ensure. C) comparison of the files. Device master record (dmr) according to 21 cfr part 820.181; What are the requirements for establishing and maintaining mdr files or records that apply to me? The device history record (dhr), device master record (dmr), and. Device History Record Mdr.
From criticalthinking.cloud
what is an device history record Device History Record Mdr Each manufacturer shall maintain device history records (dhr's). The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Device master record (dmr) according to 21 cfr part 820.181; Device history record (dhr) according to 21 cfr part 820.184; C) comparison of the files. The medical device reporting (mdr) regulation (21 cfr part 803). Device History Record Mdr.
From www.arenasolutions.com
Device Master Record (DMR) Definition Arena Device History Record Mdr C) comparison of the files. What are the requirements for establishing and maintaining mdr files or records that apply to me? The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance.. Device History Record Mdr.
From medicaldevices.freyrsolutions.com
What is Medical Device Reporting (MDR)? Freyr Medical Devices Device History Record Mdr Each manufacturer shall maintain device history records (dhr's). Device history record (dhr) according to 21 cfr part 820.184; Device master record (dmr) according to 21 cfr part 820.181; C) comparison of the files. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. The. Device History Record Mdr.
From www.greenlight.guru
Medical Device Reporting (MDR) How to Take Advantage of Your Device History Record Mdr What are the requirements for establishing and maintaining mdr files or records that apply to me? Device history record (dhr) according to 21 cfr part 820.184; Each manufacturer shall maintain device history records (dhr's). The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The device history record (dhr), device master record (dmr),. Device History Record Mdr.
From www.arenasolutions.com
Device History Record (DHR) Definition Arena Device History Record Mdr Each manufacturer shall maintain device history records (dhr's). Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device reporting (mdr) regulation (21 cfr part. Device History Record Mdr.
From www.slideserve.com
PPT Design documentation PowerPoint Presentation, free download ID Device History Record Mdr C) comparison of the files. Each manufacturer shall establish and maintain procedures to ensure. Device history record (dhr) according to 21 cfr part 820.184; The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical. Device History Record Mdr.
From www.youtube.com
Device Master Record & Device History Record A Regulatory YouTube Device History Record Mdr The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. What are the requirements for establishing and maintaining mdr files or records that apply to me? The medical device reporting (mdr). Device History Record Mdr.
From www.instantgmp.com
Device History Record Device History Record Mdr C) comparison of the files. Each manufacturer shall establish and maintain procedures to ensure. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. What are the requirements for establishing and maintaining mdr files or records that apply to me? The device history record. Device History Record Mdr.
From www.qualitymeddev.com
Device History Record (DHR) An Overview QualityMedDev Device History Record Mdr Device master record (dmr) according to 21 cfr part 820.181; The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. What are the requirements for establishing and maintaining mdr files or records that apply to me? Each manufacturer shall maintain device history records (dhr's). Maintaining a complete and accurate design history file (dhf). Device History Record Mdr.
From www.youtube.com
Design History File (DHF), the Device Master Record (DMR) and the Device History Record Mdr What are the requirements for establishing and maintaining mdr files or records that apply to me? The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. Device master record (dmr) according to 21 cfr part 820.181; Maintaining a complete and accurate design history file (dhf) is mandatory for. Device History Record Mdr.
From www.vrogue.co
Device Master Records Design History Files Gmp Docs vrogue.co Device History Record Mdr The device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. C) comparison of the files. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers,. Device History Record Mdr.
From www.todaysmedicaldevelopments.com
Medical device industry Leveraging the digital thread Today's Device History Record Mdr Device master record (dmr) according to 21 cfr part 820.181; Each manufacturer shall maintain device history records (dhr's). What are the requirements for establishing and maintaining mdr files or records that apply to me? Device history record (dhr) according to 21 cfr part 820.184; Each manufacturer shall establish and maintain procedures to ensure. The medical device reporting (mdr) regulation (21. Device History Record Mdr.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Device History Record Mdr Each manufacturer shall establish and maintain procedures to ensure. Each manufacturer shall maintain device history records (dhr's). The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Device master record (dmr) according to 21 cfr part 820.181; The device history record (dhr), device master record (dmr), and design history file (dhf) are the. Device History Record Mdr.
From www.youtube.com
Device History Record YouTube Device History Record Mdr What are the requirements for establishing and maintaining mdr files or records that apply to me? Device history record (dhr) according to 21 cfr part 820.184; Each manufacturer shall establish and maintain procedures to ensure. C) comparison of the files. Each manufacturer shall maintain device history records (dhr's). Maintaining a complete and accurate design history file (dhf) is mandatory for. Device History Record Mdr.
From docs.oracle.com
Oracle Manufacturing Implementing Oracle ERecords in Discrete Device History Record Mdr Each manufacturer shall maintain device history records (dhr's). Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. Each manufacturer shall establish and maintain procedures to ensure. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and.. Device History Record Mdr.
From www.presentationeze.com
Device History Record (DHR) PresentationEZE Device History Record Mdr Each manufacturer shall maintain device history records (dhr's). C) comparison of the files. Maintaining a complete and accurate design history file (dhf) is mandatory for medical device manufacturers under fda and mdr regulations, acting as a definitive record of. What are the requirements for establishing and maintaining mdr files or records that apply to me? Device master record (dmr) according. Device History Record Mdr.