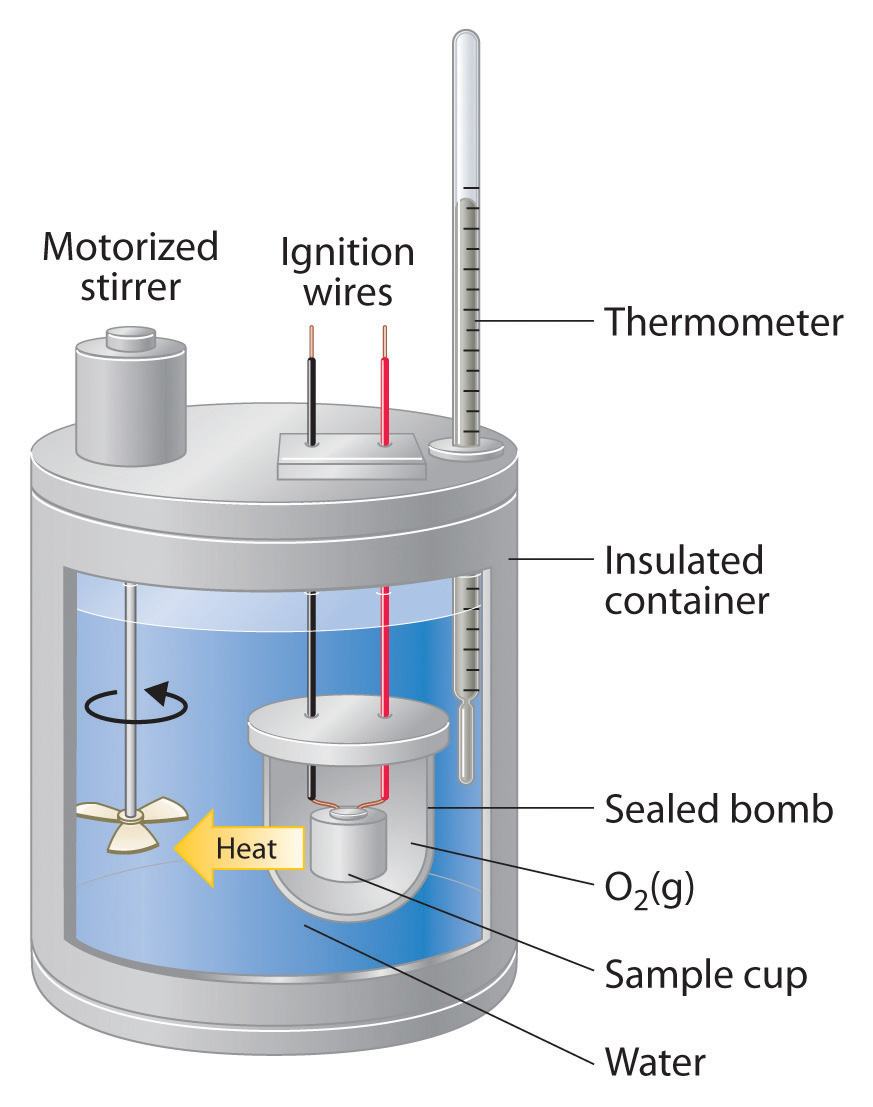
Bomb Calorimetry Value . There are two basic types of calorimeters: Gross calorific value (g.c.v) or higher calorific value (h.c.v): Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the.
from saylordotorg.github.io
There are two basic types of calorimeters: Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Gross calorific value (g.c.v) or higher calorific value (h.c.v): Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature.
Calorimetry
Bomb Calorimetry Value Gross calorific value (g.c.v) or higher calorific value (h.c.v): Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. There are two basic types of calorimeters: Gross calorific value (g.c.v) or higher calorific value (h.c.v): Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels.
From docslib.org
Bomb Calorimetry Heat of Combustion of Naphthalene Most Tabulated ∆H Bomb Calorimetry Value Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. There are two basic types of calorimeters: Bomb calorimetry is generally used to measure the heat of combustion for organic materials. Gross. Bomb Calorimetry Value.
From www.youtube.com
3Bomb Calorimeter II Calorific Value II HCV II Fuels YouTube Bomb Calorimetry Value There are two basic types of calorimeters: Bomb calorimetry is generally used to measure the heat of combustion for organic materials. Gross calorific value (g.c.v) or higher calorific value (h.c.v): A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. It is total quantity of heat liberated when one unit of fuel is burnt. Bomb Calorimetry Value.
From www.youtube.com
Bomb calorimeter, calculation of calorific value,HCV or GCV, correction Bomb Calorimetry Value Gross calorific value (g.c.v) or higher calorific value (h.c.v): A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate. Bomb Calorimetry Value.
From www.glomro.com
Powerful Oxygen Bomb Calorimeter , Full Auto Calorific Value Measuring Bomb Calorimetry Value Gross calorific value (g.c.v) or higher calorific value (h.c.v): A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. There are two basic types of calorimeters: Thus the term calorific value (or heat of combustion) as measured. Bomb Calorimetry Value.
From www.youtube.com
Bomb Calorimeter_Methodology and Solved Numerical _Fuel and its Bomb Calorimetry Value Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. Gross calorific value (g.c.v) or higher calorific value (h.c.v): A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. There are two basic types of calorimeters: Describe a simple calorimeter and explain how. Bomb Calorimetry Value.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download Bomb Calorimetry Value Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. There are two basic types of calorimeters: It is total quantity of heat liberated when one. Bomb Calorimetry Value.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Bomb Calorimetry Value Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. There are two basic types of calorimeters: Bomb calorimetry is generally used to measure the heat of combustion for organic materials. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A. Bomb Calorimetry Value.
From www.youtube.com
Class 11 chemistry Thermodynamics (calorific value of fuel and Bomb Bomb Calorimetry Value It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and. Bomb Calorimetry Value.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Bomb Calorimetry Value A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. It is total quantity of heat liberated when one unit of fuel is. Bomb Calorimetry Value.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimetry Value Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by. Bomb Calorimetry Value.
From www.studocu.com
Determination of calorific value by Bomb Calorimeter Experiment A Bomb Calorimetry Value Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. There are two basic types of. Bomb Calorimetry Value.
From www.youtube.com
Ultimate Analysis of Coal Calorific Value Bomb Calorimeter Bomb Calorimetry Value There are two basic types of calorimeters: Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. Bomb calorimetry is generally used to measure the heat. Bomb Calorimetry Value.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimetry Value Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. There are two basic types of calorimeters: Describe a simple calorimeter and explain. Bomb Calorimetry Value.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimetry Value Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. Thus the term calorific value (or heat of combustion) as measured. Bomb Calorimetry Value.
From saylordotorg.github.io
Calorimetry Bomb Calorimetry Value There are two basic types of calorimeters: Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. Bomb calorimetry is generally used to measure the heat of. Bomb Calorimetry Value.
From www.youtube.com
Bomb Calorimeter Experiment Calorific Value of Solid Fuel by Bomb Bomb Calorimetry Value Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. Dulong’s formula used to calculate the theoretical calorific value of fuel if the. Bomb Calorimetry Value.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimetry Value Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels.. Bomb Calorimetry Value.
From wiringdiagram99.blogspot.com
Which Diagram Is A Bomb Calorimeter Wiring Diagram Database Bomb Calorimetry Value Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. It is total quantity of heat. Bomb Calorimetry Value.
From www.alamy.com
Cross section of a typical bomb calorimeter Stock Photo 24063705 Alamy Bomb Calorimetry Value Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. A bomb calorimeter is used for finding the higher calorific value. Bomb Calorimetry Value.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Bomb Calorimetry Value Gross calorific value (g.c.v) or higher calorific value (h.c.v): There are two basic types of calorimeters: It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Thus the. Bomb Calorimetry Value.
From www.youtube.com
Calorific value check for petroleum Oil Through the help of bomb Bomb Calorimetry Value There are two basic types of calorimeters: Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. Gross calorific value (g.c.v) or higher calorific value (h.c.v): Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Bomb calorimetry is generally. Bomb Calorimetry Value.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Bomb Calorimetry Value It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. Bomb calorimetry is generally used to measure the heat of combustion. Bomb Calorimetry Value.
From chinainstrument.en.made-in-china.com
ASTM D240 Automatic Calorific Value Bomb Calorimeter Bomb Calorimeter Bomb Calorimetry Value It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. There are two basic types of calorimeters: Gross calorific value (g.c.v) or higher calorific value (h.c.v): Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat. Bomb Calorimetry Value.
From www.youtube.com
calorific value by using bomb calorimeter YouTube Bomb Calorimetry Value Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. There are two basic types of calorimeters: Gross calorific value (g.c.v) or higher calorific value (h.c.v): It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. Bomb. Bomb Calorimetry Value.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimetry Value Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled. Bomb Calorimetry Value.
From www.youtube.com
Determination of calorific value of solid/liquid fuel using Bomb Bomb Calorimetry Value Gross calorific value (g.c.v) or higher calorific value (h.c.v): Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. It is total quantity of heat liberated when one unit of fuel is burnt. Bomb Calorimetry Value.
From www.labxyi.com
ASTM D240 Bomb Calorimeter Calorific value tester Bomb Calorimetry Value It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. Gross calorific value (g.c.v) or higher calorific value (h.c.v): A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Describe a simple calorimeter and explain how it is. Bomb Calorimetry Value.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimetry Value Gross calorific value (g.c.v) or higher calorific value (h.c.v): Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. There are two basic types of calorimeters: It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has. Bomb Calorimetry Value.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Bomb Calorimetry Value Bomb calorimetry is generally used to measure the heat of combustion for organic materials. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. There are two basic types of calorimeters: It is. Bomb Calorimetry Value.
From www.studypool.com
SOLUTION Bomb calorimeter study material Studypool Bomb Calorimetry Value A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled to room temperature. Gross calorific value (g.c.v) or higher calorific value (h.c.v): Thus the term calorific value (or heat of combustion). Bomb Calorimetry Value.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation ID3206969 Bomb Calorimetry Value A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. There are two basic types of calorimeters: Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is. Bomb Calorimetry Value.
From lms.msitonline.org
ME4THYR Exp 3. Determination of Calorific value of a solid fuel by Bomb Calorimetry Value Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. Describe a simple calorimeter and explain how. Bomb Calorimetry Value.
From www.youtube.com
Bomb Calorimeter I Determination of calorific value of solid & liquid Bomb Calorimetry Value Bomb calorimetry is generally used to measure the heat of combustion for organic materials. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary. It is total quantity of heat liberated when one unit of fuel is burnt completely and the product of combustion has been cooled. Bomb Calorimetry Value.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimetry Value Thus the term calorific value (or heat of combustion) as measured in a bomb calorimeter denotes the heat liberated by the. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Gross calorific value (g.c.v) or higher calorific value (h.c.v): There are two basic types of calorimeters: Describe a simple calorimeter and explain how. Bomb Calorimetry Value.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimetry Value A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. There are two basic types of calorimeters: Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific. Bomb Calorimetry Value.