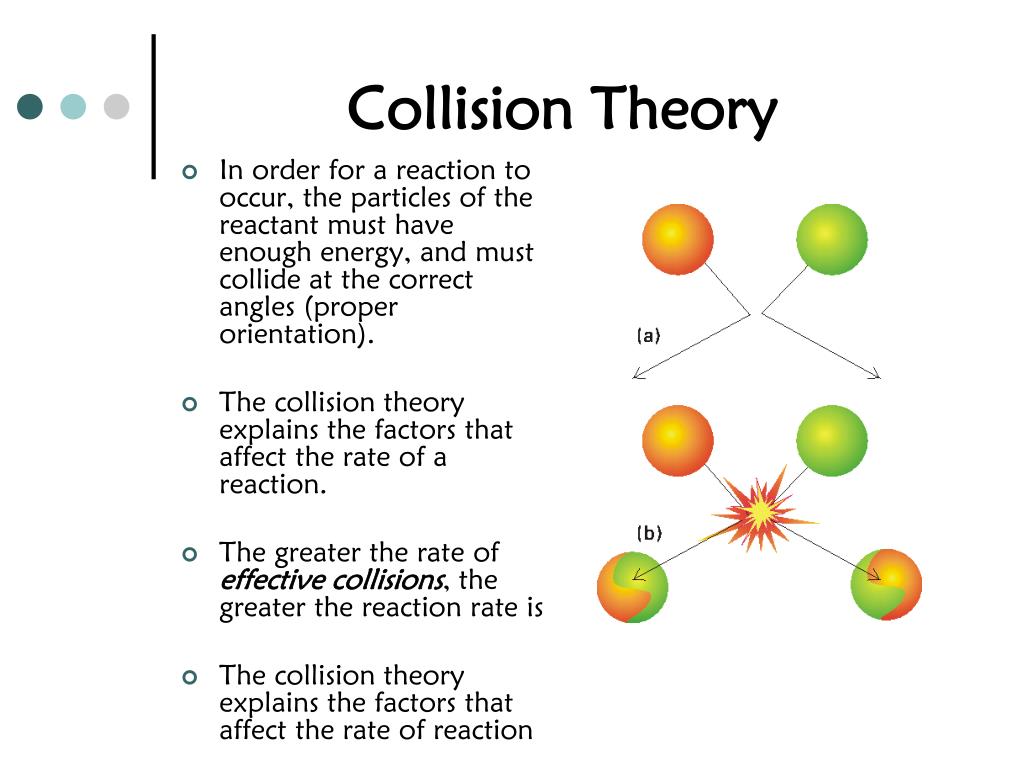
Collision Effect In . this page describes the collision theory of reaction rates. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. It concentrates on the key things which decide whether a particular. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. The rate of a reaction is proportional to the rate of reactant. collision theory provides a simple but effective explanation for the effect of many experimental parameters. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory is based on the following postulates: collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction.
from learningschoolbrautantkn.z22.web.core.windows.net
collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory provides a simple but effective explanation for the effect of many experimental parameters. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. this page describes the collision theory of reaction rates. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory is based on the following postulates: The rate of a reaction is proportional to the rate of reactant. It concentrates on the key things which decide whether a particular.
Collision Theory Grade 12 Pdf
Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters. this page describes the collision theory of reaction rates. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory provides a simple but effective explanation for the effect of many experimental parameters. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory is based on the following postulates: The rate of a reaction is proportional to the rate of reactant. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. It concentrates on the key things which decide whether a particular.
From www.sciencefacts.net
Inelastic Collision Definition, Formula, and Examples Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters. The rate of a reaction is proportional to the rate of reactant. collision theory is based on the following postulates: collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory explains why. Collision Effect In.
From www.youtube.com
Speed of reactions effective collisions YouTube Collision Effect In collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. this page describes the collision theory of reaction rates. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory provides a simple but effective explanation for the effect. Collision Effect In.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Effect In The rate of a reaction is proportional to the rate of reactant. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. this page describes the collision theory of reaction rates.. Collision Effect In.
From philschatz.com
Photon Momentum · Physics Collision Effect In collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory provides a simple but effective explanation for the effect of many experimental parameters. The rate of a reaction is. Collision Effect In.
From www.pinterest.com
Simple Collision Theory Collision theory, Theories, Energy activities Collision Effect In this page describes the collision theory of reaction rates. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. The rate of a reaction is proportional to the rate of. Collision Effect In.
From physics.educour.in
Elastic and Inelastic Collision Class 11 CBSE CBSE Physics Collision Effect In this page describes the collision theory of reaction rates. collision theory is based on the following postulates: collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction.. Collision Effect In.
From studylib.net
Elastic and Inelastic Collisions Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. It concentrates on the key things which decide whether a particular. this page describes the collision theory of reaction rates. collision theory is based on the following postulates: The rate of a reaction is proportional to the rate of reactant.. Collision Effect In.
From www.sciencefacts.net
Inelastic Collision Definition, Formula, and Examples Collision Effect In use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory is based on the following postulates: collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. It concentrates on the key things which decide whether a particular. collision theory explains. Collision Effect In.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Effect In It concentrates on the key things which decide whether a particular. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. The rate of a reaction is proportional to. Collision Effect In.
From www.savemyexams.com
Collision Theory Cambridge O Level Chemistry Revision Notes 2023 Collision Effect In use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory is based on the following postulates: collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory provides a simple but effective explanation for the effect of. Collision Effect In.
From www.slideserve.com
PPT Unit 9 Reaction Rates and Equilibrium PowerPoint Presentation Collision Effect In collision theory is based on the following postulates: The rate of a reaction is proportional to the rate of reactant. collision theory provides a simple but effective explanation for the effect of many experimental parameters. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory explains why. Collision Effect In.
From quizizz.com
Collision Theory and Reaction Rate Science Quizizz Collision Effect In collision theory is based on the following postulates: use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. The rate of a reaction is proportional to the rate of reactant. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction.. Collision Effect In.
From scienceres-edcp-educ.sites.olt.ubc.ca
Collisions MSTLTT Collision Effect In use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. this page describes. Collision Effect In.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID1991748 Collision Effect In It concentrates on the key things which decide whether a particular. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory provides a simple but effective. Collision Effect In.
From www.youtube.com
Collision theory in the rate of reaction YouTube Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory explains why different. Collision Effect In.
From www.slideserve.com
PPT Chapter 18 Chemical Equilibrium PowerPoint Presentation, free Collision Effect In collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. It concentrates on the key things which decide whether a particular. The rate of a reaction is proportional to the rate of reactant. collision theory explains why different reactions occur at different rates, and suggests ways to change. Collision Effect In.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision Effect In collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. The rate of a reaction is proportional to the rate of reactant. collision theory explains why different reactions occur at different. Collision Effect In.
From www.youtube.com
Factors Affecting Rate of Reaction + Collision Theory GCSE Science Collision Effect In use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. It concentrates on the key things which decide whether a particular. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. collision theory explains why different reactions occur at different. Collision Effect In.
From learningschoolbrautantkn.z22.web.core.windows.net
Collision Theory Grade 12 Pdf Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters. The rate of a reaction is proportional to the rate of reactant. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. collision theory is based on the following postulates: collision. Collision Effect In.
From mahonefirm.com
The 7 Most Common Causes of a Collision The Mahone Firm Collision Effect In use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory provides a simple but effective explanation for the effect of many experimental parameters. this page describes the collision theory of. Collision Effect In.
From www.slideserve.com
PPT The Procedure of Collision Detection PowerPoint Presentation Collision Effect In It concentrates on the key things which decide whether a particular. The rate of a reaction is proportional to the rate of reactant. collision theory provides a simple but effective explanation for the effect of many experimental parameters. this page describes the collision theory of reaction rates. use the postulates of collision theory to explain the effects. Collision Effect In.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.9.1 Collision Theory & Rates翰林国际教育 Collision Effect In collision theory is based on the following postulates: collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. this page describes the collision theory of reaction rates.. Collision Effect In.
From www.researchgate.net
Exaggerated collision effect between two particles Download Collision Effect In this page describes the collision theory of reaction rates. collision theory is based on the following postulates: collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. It concentrates. Collision Effect In.
From www.youtube.com
Collision Theory / Reaction Rate YouTube Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. collision theory is based on the following postulates: use the postulates of collision theory to explain the effects of. Collision Effect In.
From courses.lumenlearning.com
Collision Theory Chemistry Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters. It concentrates on the key things which decide whether a particular. this page describes the collision theory of reaction rates. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory explains why. Collision Effect In.
From www.ck12.org
Collision Theory CK12 Foundation Collision Effect In It concentrates on the key things which decide whether a particular. collision theory provides a simple but effective explanation for the effect of many experimental parameters. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. this page describes the collision theory of reaction rates. collision theory is based. Collision Effect In.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 Collision Effect In collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. It concentrates on the key things which decide whether a particular. this page describes the collision theory of reaction rates. The. Collision Effect In.
From integratedsciencegeneral11.weebly.com
Collision Theory Explains Chemical Reactions Integrated Science 11 Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory provides a simple but effective explanation for the effect of many experimental parameters. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory is based on. Collision Effect In.
From thechemistrynotes.com
Collision Theory of Chemical Collision Effect In It concentrates on the key things which decide whether a particular. this page describes the collision theory of reaction rates. The rate of a reaction is proportional to the rate of reactant. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. collision theory explains why different. Collision Effect In.
From www.slideserve.com
PPT Momentum and Collisions PowerPoint Presentation, free download Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. . Collision Effect In.
From www.scribd.com
Chapter 3 Example of 2D Collision Collision Momentum Collision Effect In collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a. collision theory is based on the following postulates: collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. use the postulates of collision theory to explain the. Collision Effect In.
From www.freepik.com
Realistic two lights collision effect Free Vector Collision Effect In use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory provides a simple but effective explanation for the effect of many experimental parameters. collision theory is based on the following postulates: collision theory explains why different reactions occur at different rates, and suggests ways to change the. Collision Effect In.
From physics.educour.in
Elastic and Inelastic Collision Class 11 CBSE CBSE Physics Collision Effect In this page describes the collision theory of reaction rates. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory provides a simple but effective explanation for the effect of many experimental parameters. The rate of a reaction is proportional to the rate of reactant. collision theory is. Collision Effect In.
From 2012books.lardbucket.org
The Collision Model of Chemical Collision Effect In use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. It concentrates on the key things which decide whether a particular. collision theory provides a simple but effective explanation for the effect of many experimental parameters. collision theory explains why different reactions occur at different rates, and suggests ways to. Collision Effect In.
From lambdageeks.com
15 Elastic Collision Examples Detailed Facts And FAQs LAMBDAGEEKS Collision Effect In collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. this page describes the collision theory of reaction rates. collision theory provides a simple but effective explanation for the effect of many experimental parameters. collision theory explains why different reactions occur at different rates, and suggests ways to change. Collision Effect In.