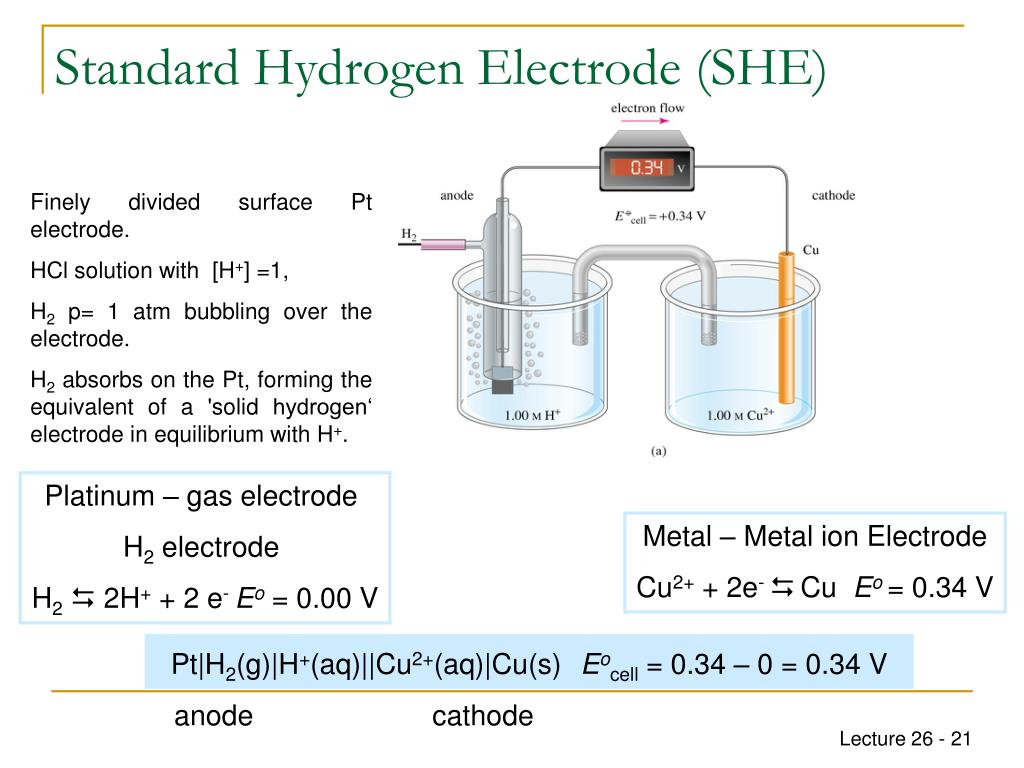
Nernst Equation For Standard Hydrogen Electrode . for the standard cell potential, relative to a given refer ence. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). U = δφ − δφ standard. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. For aqueous electrolytes, the usual reference electrode is the. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode.
from exoyfxrvd.blob.core.windows.net
For aqueous electrolytes, the usual reference electrode is the. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. U = δφ − δφ standard. for the standard cell potential, relative to a given refer ence. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she).
Standard Hydrogen Electrode Nernst Equation at Silva Upchurch blog
Nernst Equation For Standard Hydrogen Electrode for the standard cell potential, relative to a given refer ence. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. For aqueous electrolytes, the usual reference electrode is the. U = δφ − δφ standard. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. for the standard cell potential, relative to a given refer ence. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode.
From www.youtube.com
Standard hydrogen electrode ( SHE ) , EC CELL, NERNST EQUATION BY Nernst Equation For Standard Hydrogen Electrode this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). for the standard cell potential, relative to a given refer ence. U = δφ − δφ standard. For aqueous electrolytes, the usual reference electrode is the. the standard potential of an electrode is its interfacial voltage minus. Nernst Equation For Standard Hydrogen Electrode.
From www.toppr.com
For a cell reaction M^H + (aq) + ne^ → M(s) , the Nernst equation Nernst Equation For Standard Hydrogen Electrode hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. For aqueous electrolytes, the usual reference electrode is the. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). the nernst equation relates the effective concentrations (activities). Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Electrochemistry 9 Nernst Equation Find Electrode Potential or EMF Nernst Equation For Standard Hydrogen Electrode hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. for the standard cell potential, relative to a given refer ence. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). the nernst equation relates the. Nernst Equation For Standard Hydrogen Electrode.
From www.slideserve.com
PPT The Nernst Equation PowerPoint Presentation, free download ID Nernst Equation For Standard Hydrogen Electrode this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. U = δφ − δφ standard. the nernst equation relates the effective concentrations (activities) of the components of a cell. Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
CLASS 12 JEE & NEET ELECTROCHEMISTRY NERNST EQUATION & ELECTRODE Nernst Equation For Standard Hydrogen Electrode U = δφ − δφ standard. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode. Nernst Equation For Standard Hydrogen Electrode.
From mungfali.com
Ppt Chapter 17 Electrochemistry Powerpoint Presentation, Free A12 Nernst Equation For Standard Hydrogen Electrode the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. this. Nernst Equation For Standard Hydrogen Electrode.
From greenenergymaterial.com
Nernst Equation Electrochemistry Electrolysis Nernst Equation For Standard Hydrogen Electrode For aqueous electrolytes, the usual reference electrode is the. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). the nernst equation relates the effective concentrations (activities). Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Nernst EquationMeasurement of Electrode Potential LN 8 CLASS XII Nernst Equation For Standard Hydrogen Electrode U = δφ − δφ standard. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). For aqueous electrolytes, the usual reference electrode is the. the nernst. Nernst Equation For Standard Hydrogen Electrode.
From en.ppt-online.org
Electrochemistry. Oxidationreduction equilibrium in water solutions Nernst Equation For Standard Hydrogen Electrode U = δφ − δφ standard. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. For aqueous electrolytes, the usual reference electrode is the. the nernst equation relates the. Nernst Equation For Standard Hydrogen Electrode.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID6600057 Nernst Equation For Standard Hydrogen Electrode the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). for the standard cell potential, relative to a given refer ence. the nernst equation relates the effective concentrations (activities). Nernst Equation For Standard Hydrogen Electrode.
From exoyfxrvd.blob.core.windows.net
Standard Hydrogen Electrode Nernst Equation at Silva Upchurch blog Nernst Equation For Standard Hydrogen Electrode for the standard cell potential, relative to a given refer ence. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. this is the nernst equation for the. Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Nernst Equation ELECTROCHEMISTRY Class 12 Nernst equation and Nernst Equation For Standard Hydrogen Electrode this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). U = δφ − δφ standard. For aqueous electrolytes, the usual reference electrode is the. for the standard cell potential, relative to a given refer ence. the standard potential of an electrode is its interfacial voltage minus. Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Standard hydrogen electrode and Nernst equation problems YouTube Nernst Equation For Standard Hydrogen Electrode U = δφ − δφ standard. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). for the standard cell potential, relative to a given refer ence. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. the nernst. Nernst Equation For Standard Hydrogen Electrode.
From exoyfxrvd.blob.core.windows.net
Standard Hydrogen Electrode Nernst Equation at Silva Upchurch blog Nernst Equation For Standard Hydrogen Electrode for the standard cell potential, relative to a given refer ence. For aqueous electrolytes, the usual reference electrode is the. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. hydrogen’s. Nernst Equation For Standard Hydrogen Electrode.
From itsreleased.com
Nernst Equation Understanding Electrochemical Equilibrium Nernst Equation For Standard Hydrogen Electrode hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. For aqueous electrolytes, the usual reference electrode is the. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. for the standard cell potential, relative to a given refer. Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Calculate the potential of hydrogen electrode in contact with a Nernst Equation For Standard Hydrogen Electrode the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. U = δφ − δφ standard. For aqueous electrolytes, the usual reference electrode is the. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. this is the nernst equation. Nernst Equation For Standard Hydrogen Electrode.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Nernst Equation For Standard Hydrogen Electrode U = δφ − δφ standard. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. this is the nernst equation for the (equilibrium) electrode potential if the reference. Nernst Equation For Standard Hydrogen Electrode.
From exomimdmo.blob.core.windows.net
Representation Of Standard Hydrogen Electrode at Rona Loomis blog Nernst Equation For Standard Hydrogen Electrode this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). For aqueous electrolytes, the usual reference electrode is the. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. hydrogen’s standard electrode potential (e ) is taken to be. Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Electrode Potential ll Nernst equation ll Standard hydrogen electrode Nernst Equation For Standard Hydrogen Electrode the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. For aqueous electrolytes, the usual reference electrode is the. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. U = δφ − δφ standard. the nernst equation relates the. Nernst Equation For Standard Hydrogen Electrode.
From exoyfxrvd.blob.core.windows.net
Standard Hydrogen Electrode Nernst Equation at Silva Upchurch blog Nernst Equation For Standard Hydrogen Electrode for the standard cell potential, relative to a given refer ence. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. For aqueous electrolytes, the usual reference electrode is the. U = δφ − δφ standard. this is the nernst equation for the (equilibrium) electrode potential if the reference. Nernst Equation For Standard Hydrogen Electrode.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 9 Nernst Equation PowerPoint Nernst Equation For Standard Hydrogen Electrode hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. U = δφ − δφ standard. For aqueous electrolytes, the usual reference electrode is the. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. the standard potential of. Nernst Equation For Standard Hydrogen Electrode.
From www.slideserve.com
PPT Electrochemistry Part IV Spontaneity & Nernst Equation Nernst Equation For Standard Hydrogen Electrode U = δφ − δφ standard. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. For aqueous electrolytes, the usual reference electrode is the. hydrogen’s standard electrode potential (e ) is. Nernst Equation For Standard Hydrogen Electrode.
From www.toppr.com
For a cell reaction M^H + (aq) + ne^ → M(s) , the Nernst equation Nernst Equation For Standard Hydrogen Electrode hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. for the standard cell potential, relative to a given refer ence. For aqueous electrolytes, the usual reference electrode is the. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode.. Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Electrochemistry Lec 2 Standard Hydrogen Electrode Types of Nernst Equation For Standard Hydrogen Electrode for the standard cell potential, relative to a given refer ence. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. For aqueous electrolytes, the usual reference electrode is the. U = δφ − δφ standard. the standard potential of an electrode is its interfacial voltage minus. Nernst Equation For Standard Hydrogen Electrode.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Nernst Equation For Standard Hydrogen Electrode the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). U = δφ − δφ standard. For aqueous electrolytes, the usual reference electrode is the. for the standard cell potential,. Nernst Equation For Standard Hydrogen Electrode.
From www.toppr.com
For a cell reaction M^H + (aq) + ne^ → M(s) , the Nernst equation Nernst Equation For Standard Hydrogen Electrode the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. For aqueous. Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Derivation of Nernst equation for a single electrode potential YouTube Nernst Equation For Standard Hydrogen Electrode the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. U = δφ − δφ standard. for the standard cell potential, relative to a given refer ence. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. the. Nernst Equation For Standard Hydrogen Electrode.
From exoyfxrvd.blob.core.windows.net
Standard Hydrogen Electrode Nernst Equation at Silva Upchurch blog Nernst Equation For Standard Hydrogen Electrode hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she).. Nernst Equation For Standard Hydrogen Electrode.
From askfilo.com
3.4. Calculate the potential of hydrogen electrode in contact with a solu.. Nernst Equation For Standard Hydrogen Electrode For aqueous electrolytes, the usual reference electrode is the. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. for the standard cell potential, relative to a given refer ence. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode. Nernst Equation For Standard Hydrogen Electrode.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID370431 Nernst Equation For Standard Hydrogen Electrode the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. For aqueous electrolytes, the usual reference electrode is the. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. this is the nernst equation for the (equilibrium) electrode potential if the reference. Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Electrochemistry Standard Hydrogen Electrode, Nernst Equation YouTube Nernst Equation For Standard Hydrogen Electrode this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. for the standard cell potential, relative to a given refer ence. the nernst equation relates the. Nernst Equation For Standard Hydrogen Electrode.
From www.numerade.com
SOLVED 1. [6 points] a. Show with the aid of a form of the Nernst Nernst Equation For Standard Hydrogen Electrode For aqueous electrolytes, the usual reference electrode is the. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). for the standard cell potential, relative to a given refer ence. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the. Nernst Equation For Standard Hydrogen Electrode.
From www.toppr.com
Write Nernst equation for single electrode potential at 298 K Nernst Equation For Standard Hydrogen Electrode the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. For aqueous electrolytes, the usual reference electrode is the. the standard potential of an electrode is its interfacial voltage minus that of a standard reference electrode. for the standard cell potential, relative to a given refer ence. this. Nernst Equation For Standard Hydrogen Electrode.
From thechemistrynotes.com
The Nernst Equation Derivation, Application, and Limitations Nernst Equation For Standard Hydrogen Electrode for the standard cell potential, relative to a given refer ence. this is the nernst equation for the (equilibrium) electrode potential if the reference electrode is the standard hydrogen electrode (she). hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. U = δφ − δφ standard.. Nernst Equation For Standard Hydrogen Electrode.
From www.youtube.com
Nernst EquationHow to find nersrt equation of a cellNernst equation Nernst Equation For Standard Hydrogen Electrode U = δφ − δφ standard. hydrogen’s standard electrode potential (e ) is taken to be zero volts at all temperatures, that is, e = 0. the nernst equation relates the effective concentrations (activities) of the components of a cell reaction to the standard. the standard potential of an electrode is its interfacial voltage minus that of. Nernst Equation For Standard Hydrogen Electrode.