Potassium Hydroxide Vs Potassium Chloride . It can be generated by treating. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. potash and potassium, while often used interchangeably in common language, have distinct differences in. there are some sodium and potassium salts that strongly differ in water solubilities. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. There are a lot of.
from www.chegg.com
there are some sodium and potassium salts that strongly differ in water solubilities. potash and potassium, while often used interchangeably in common language, have distinct differences in. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). There are a lot of. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. It can be generated by treating.
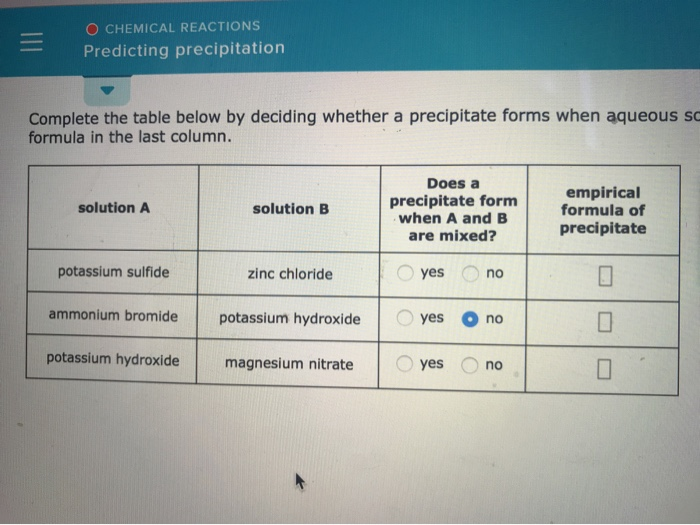
Solved O CHEMICAL REACTIONS Predicting precipitation
Potassium Hydroxide Vs Potassium Chloride whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. there are some sodium and potassium salts that strongly differ in water solubilities. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). It can be generated by treating. potash and potassium, while often used interchangeably in common language, have distinct differences in. There are a lot of. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic.
From melscience.com
Reactions of potassium and potassium hydroxide MEL Chemistry Potassium Hydroxide Vs Potassium Chloride It can be generated by treating. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺). Potassium Hydroxide Vs Potassium Chloride.
From www.elevise.co.uk
C4 J) Electrolysis Part 1 AQA Combined Science Trilogy Elevise Potassium Hydroxide Vs Potassium Chloride There are a lot of. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. a strong base is something like sodium hydroxide or potassium hydroxide. Potassium Hydroxide Vs Potassium Chloride.
From pediaa.com
Difference Between Potassium Gluconate and Potassium Chloride Potassium Hydroxide Vs Potassium Chloride It can be generated by treating. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. potash and potassium, while often used interchangeably in common language, have distinct differences in. a strong base is something like sodium hydroxide or potassium. Potassium Hydroxide Vs Potassium Chloride.
From www.coursehero.com
[Solved] Potassium chlorate to form potassium chloride and Potassium Hydroxide Vs Potassium Chloride It can be generated by treating. potash and potassium, while often used interchangeably in common language, have distinct differences in. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and. Potassium Hydroxide Vs Potassium Chloride.
From pediaa.com
Difference Between Potassium Hydroxide and Sodium Hydroxide Potassium Hydroxide Vs Potassium Chloride potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. It can be generated by treating. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. a strong base is something like sodium hydroxide or potassium hydroxide which is fully. Potassium Hydroxide Vs Potassium Chloride.
From www.youtube.com
Equation for Potassium Chloride Dissolving in Water ( KCl + H2O) YouTube Potassium Hydroxide Vs Potassium Chloride It can be generated by treating. potash and potassium, while often used interchangeably in common language, have distinct differences in. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. there are some sodium and potassium salts that strongly differ in water solubilities. There are a lot of. the limiting molar conductances (λ. Potassium Hydroxide Vs Potassium Chloride.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Potassium Hydroxide Vs Potassium Chloride whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. there are some sodium and potassium salts that strongly differ in water solubilities. It can be generated by treating. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). potassium hydroxide is represented by the chemical. Potassium Hydroxide Vs Potassium Chloride.
From www.differencebetween.com
Difference Between Calcium Chloride and Potassium Chloride Compare Potassium Hydroxide Vs Potassium Chloride the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. there are some sodium and potassium salts that strongly differ in water solubilities.. Potassium Hydroxide Vs Potassium Chloride.
From www.differencebetween.com
Difference Between Potassium Acetate and Potassium Chloride Compare Potassium Hydroxide Vs Potassium Chloride potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. It can be generated by treating. potash and potassium, while often used interchangeably in common language, have distinct differences in. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide.. Potassium Hydroxide Vs Potassium Chloride.
From pharmabeej.com
What Is The Molecular Formula Of Potassium Hydroxide? Pharmabeej Potassium Hydroxide Vs Potassium Chloride potash and potassium, while often used interchangeably in common language, have distinct differences in. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. There are a lot of. . Potassium Hydroxide Vs Potassium Chloride.
From www.difference.wiki
Potassium Chloride vs. Potassium Phosphate What’s the Difference? Potassium Hydroxide Vs Potassium Chloride the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). there are some sodium and potassium salts that strongly differ in water solubilities. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide.. Potassium Hydroxide Vs Potassium Chloride.
From testbook.com
Potassium Hydroxide Learn Definition, Symbol, Preparation here Potassium Hydroxide Vs Potassium Chloride whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potash and potassium, while often used interchangeably in common language, have distinct differences in. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). It can be generated by treating. there are some sodium and potassium. Potassium Hydroxide Vs Potassium Chloride.
From www.askdifference.com
Potassium Hydroxide vs. Sodium Hydroxide — What’s the Difference? Potassium Hydroxide Vs Potassium Chloride there are some sodium and potassium salts that strongly differ in water solubilities. It can be generated by treating. There are a lot of. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). potassium chloride. Potassium Hydroxide Vs Potassium Chloride.
From slideplayer.com
Binary Covalent Ionic (I) Ionic (II) ppt download Potassium Hydroxide Vs Potassium Chloride There are a lot of. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. potash and potassium, while often used interchangeably in common language, have distinct differences in. It can be generated by treating. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting. Potassium Hydroxide Vs Potassium Chloride.
From www.slideserve.com
PPT Binary Molecular PowerPoint Presentation, free download ID4566789 Potassium Hydroxide Vs Potassium Chloride potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. there are some sodium and potassium salts that strongly differ in water solubilities. the. Potassium Hydroxide Vs Potassium Chloride.
From www.differencebetween.com
Difference Between Sodium Chloride and Potassium Chloride Compare the Potassium Hydroxide Vs Potassium Chloride a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. potash and potassium, while often used interchangeably in common language, have distinct differences in. It can be generated by treating. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). potassium hydroxide is represented. Potassium Hydroxide Vs Potassium Chloride.
From www.pw.live
Potassium Hydroxide Formula, Structure, Properties, Uses Potassium Hydroxide Vs Potassium Chloride potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. there are some sodium and potassium salts that strongly differ in water solubilities. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1).. Potassium Hydroxide Vs Potassium Chloride.
From pharmabeej.com
Preparation Of 1N Ethanolic Potassium Hydroxide (KOH) Pharmabeej Potassium Hydroxide Vs Potassium Chloride the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). It can be generated by treating. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. potash and potassium, while often used interchangeably in common language,. Potassium Hydroxide Vs Potassium Chloride.
From myloview.com
Potassium hydroxide chemical structure. vector illustration. posters Potassium Hydroxide Vs Potassium Chloride potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. It can be generated by treating. the limiting molar conductances (λ 0) and ion association constants of dilute. Potassium Hydroxide Vs Potassium Chloride.
From formulasense.com
What is Potassium Hydroxide? — Formula Sense Potassium Hydroxide Vs Potassium Chloride potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. potash and potassium, while often used interchangeably in common language, have distinct differences in. It can be generated by treating. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potassium hydroxide is represented by the chemical formula koh and. Potassium Hydroxide Vs Potassium Chloride.
From www.dreamstime.com
Potassium Hydroxide, Potassium Carbonate and Sodium Chloride Stock Potassium Hydroxide Vs Potassium Chloride a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. It can be generated by treating. potash and potassium, while often used interchangeably in common language, have distinct differences in. There are a lot of. there are some sodium and potassium salts that strongly differ in water solubilities. whereas potassium chloride,. Potassium Hydroxide Vs Potassium Chloride.
From fitnessology.net
Potassium Chloride vs. Potassium Citrate Fitnessology Potassium Hydroxide Vs Potassium Chloride a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). there are some sodium and potassium salts that strongly differ in water solubilities. potassium chloride is inexpensively available and is rarely prepared intentionally in. Potassium Hydroxide Vs Potassium Chloride.
From www.britannica.com
Potassium chloride Definition, Formula, Uses, Flame Color, & Facts Potassium Hydroxide Vs Potassium Chloride whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. there are some sodium and potassium salts that strongly differ in. Potassium Hydroxide Vs Potassium Chloride.
From allschoolabs.com
Buy Here Potassium Hydroxide Allschoolabs Online Potassium Hydroxide Vs Potassium Chloride there are some sodium and potassium salts that strongly differ in water solubilities. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. It can be generated by treating. a strong base is something like sodium hydroxide or potassium hydroxide which is fully. Potassium Hydroxide Vs Potassium Chloride.
From www.dreamstime.com
Selective Focus of a Bottle of Pure Sodium Hydroxide and Potassium Potassium Hydroxide Vs Potassium Chloride a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. There are a lot of. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions. Potassium Hydroxide Vs Potassium Chloride.
From www.dreamstime.com
Potassium Hydroxide, Potassium Carbonate and Sodium Chloride Stock Potassium Hydroxide Vs Potassium Chloride There are a lot of. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potash and potassium, while often used interchangeably in common language, have distinct differences in. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. . Potassium Hydroxide Vs Potassium Chloride.
From ar.inspiredpencil.com
Potassium Hydroxide Structure Potassium Hydroxide Vs Potassium Chloride the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). potash and potassium, while often used interchangeably in common language, have distinct differences in. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. there are some sodium and potassium salts that strongly differ in water. Potassium Hydroxide Vs Potassium Chloride.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Potassium Hydroxide Vs Potassium Chloride potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions (k⁺) and hydroxide ions. There are a lot of. potash and potassium, while often used interchangeably in common language, have distinct differences in. a strong base is something like sodium hydroxide or potassium hydroxide which is fully. Potassium Hydroxide Vs Potassium Chloride.
From stock.adobe.com
Formula of potassium hydroxide. Chemical structure of potassium Potassium Hydroxide Vs Potassium Chloride the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. There are a lot of. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. It can be generated by treating. . Potassium Hydroxide Vs Potassium Chloride.
From animalia-life.club
Lewis Structure For Potassium Potassium Hydroxide Vs Potassium Chloride a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. There are a lot of. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions. Potassium Hydroxide Vs Potassium Chloride.
From hcs-lab.com
Potassium Hydroxide HCS Scientific & Chemical Pte Ltd Potassium Hydroxide Vs Potassium Chloride It can be generated by treating. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. There are a lot of. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. potassium hydroxide is represented by the chemical formula koh and it has a simple ionic structure consisting of potassium ions. Potassium Hydroxide Vs Potassium Chloride.
From noahchemicals.com
Side by Side Comparison Potassium Hydroxide and Sodium Hydroxide Potassium Hydroxide Vs Potassium Chloride potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. It can be generated by treating. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. potash and potassium, while often used interchangeably in common. Potassium Hydroxide Vs Potassium Chloride.
From chemistry.stackexchange.com
chemistry Solubility of potassium nitrate and potassium Potassium Hydroxide Vs Potassium Chloride whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. It can be generated by treating. the limiting molar conductances (λ 0) and ion association. Potassium Hydroxide Vs Potassium Chloride.
From www.askdifference.com
Potassium Chloride vs. Potassium Phosphate — What’s the Difference? Potassium Hydroxide Vs Potassium Chloride a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. there are some sodium and potassium salts that strongly differ in water solubilities. the limiting molar conductances (λ 0) and ion association constants of dilute (<0.01 mol · kg −1). potassium chloride is inexpensively available and is rarely prepared intentionally in. Potassium Hydroxide Vs Potassium Chloride.
From www.pinterest.com
Sodium vs Potassium Understanding the Differences Potassium Hydroxide Vs Potassium Chloride potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. a strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. There are a lot of. whereas potassium chloride, a more costly compound, is used to produce potassium hydroxide. there are some sodium and potassium salts that strongly differ. Potassium Hydroxide Vs Potassium Chloride.