Chlorine Element Oxidation State . chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. The oxidation state of fluorine in chemical compounds is. The oxidation state of an atom is a measure of the degree of oxidation of an atom. enter the formula of a chemical compound to find the oxidation number of each element. chlorine is the only element to have changed oxidation state. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. A net ionic charge can be specified at the. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. However, its transition is more complicated than previously.
from chem.unc.edu
chlorine is the only element to have changed oxidation state. However, its transition is more complicated than previously. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. The oxidation state of an atom is a measure of the degree of oxidation of an atom. A net ionic charge can be specified at the. enter the formula of a chemical compound to find the oxidation number of each element. The oxidation state of fluorine in chemical compounds is.
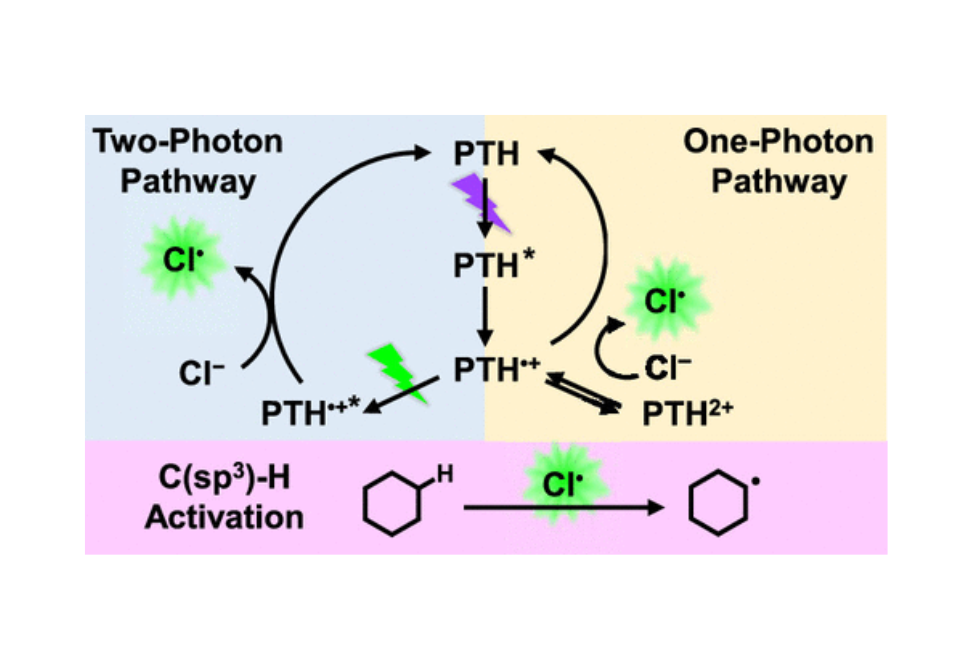
Chloride Oxidation by One or TwoPhoton Excitation of N
Chlorine Element Oxidation State the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. The oxidation state of an atom is a measure of the degree of oxidation of an atom. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. However, its transition is more complicated than previously. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. A net ionic charge can be specified at the. The oxidation state of fluorine in chemical compounds is. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is the only element to have changed oxidation state. enter the formula of a chemical compound to find the oxidation number of each element.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Chlorine Element Oxidation State The oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is the only element to have changed oxidation state. aside from the −1 oxidation states of some. Chlorine Element Oxidation State.
From www.chemistrylearner.com
Oxidation Number (State) Definition, Rules, How to Find, and Examples Chlorine Element Oxidation State chlorine is the only element to have changed oxidation state. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. The oxidation state of fluorine in chemical compounds is. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − =. Chlorine Element Oxidation State.
From sciencenotes.org
Chlorine Facts Chlorine Element Oxidation State A net ionic charge can be specified at the. chlorine is the only element to have changed oxidation state. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl −. Chlorine Element Oxidation State.
From mavink.com
Periodic Table Of Oxidation States Chlorine Element Oxidation State aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. The oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. However,. Chlorine Element Oxidation State.
From www.priyamstudycentre.com
Oxidation Number Periodic table elements Definition, Rules Chlorine Element Oxidation State The oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. A net ionic charge can be specified at the. aside from the −1 oxidation states of some chlorides, chlorine. Chlorine Element Oxidation State.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Chlorine Element Oxidation State The oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. The oxidation. Chlorine Element Oxidation State.
From www.youtube.com
How to calculate the oxidation number of Cl in HClO2 (chlorous acid Chlorine Element Oxidation State The oxidation state of fluorine in chemical compounds is. The oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is a chemical element with atomic number 17 which. Chlorine Element Oxidation State.
From mavink.com
Periodic Table Of Oxidation States Chlorine Element Oxidation State However, its transition is more complicated than previously. The oxidation state of fluorine in chemical compounds is. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. aside. Chlorine Element Oxidation State.
From infinitylearn.com
Important Topic of Chemistry Oxidation States Infinity Learn by Sri Chlorine Element Oxidation State A net ionic charge can be specified at the. The oxidation state of an atom is a measure of the degree of oxidation of an atom. However, its transition is more complicated than previously. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. the oxidation state of a monatomic. Chlorine Element Oxidation State.
From webmis.highland.cc.il.us
Classifying Chemical Reactions Chlorine Element Oxidation State chlorine is the only element to have changed oxidation state. The oxidation state of fluorine in chemical compounds is. However, its transition is more complicated than previously. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. The oxidation state of an atom is a measure. Chlorine Element Oxidation State.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Chlorine Element Oxidation State enter the formula of a chemical compound to find the oxidation number of each element. The oxidation state of an atom is a measure of the degree of oxidation of an atom. The oxidation state of fluorine in chemical compounds is. A net ionic charge can be specified at the. chlorine is a chemical element with atomic number. Chlorine Element Oxidation State.
From askfilo.com
Oxidation states of chlorine in NOCl and OCl2 are Filo Chlorine Element Oxidation State The oxidation state of fluorine in chemical compounds is. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is the only element to have changed oxidation state. However, its transition is more complicated than previously. chlorine is a chemical element with atomic number. Chlorine Element Oxidation State.
From www.nuclear-power.com
What is Chlorine Properties of Chlorine Element Symbol Cl nuclear Chlorine Element Oxidation State The oxidation state of fluorine in chemical compounds is. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. The oxidation state of an atom is a measure of. Chlorine Element Oxidation State.
From www.vectorstock.com
Atom chlorine Royalty Free Vector Image VectorStock Chlorine Element Oxidation State aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. enter the formula of a chemical compound to find the oxidation number of each element. chlorine is the only element to have changed oxidation state. the oxidation state of a monatomic ion is the same as its charge—for. Chlorine Element Oxidation State.
From educationspares.z4.web.core.windows.net
What Is Chlorine's Oxidation Number Chlorine Element Oxidation State aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. enter the formula of a chemical compound to find the oxidation number of each element. chlorine is the only element to have changed oxidation state. The oxidation state of an atom is a measure of the degree of oxidation. Chlorine Element Oxidation State.
From www.youtube.com
How to find Oxidation Numbers for Chlorine (Cl and Cl2) YouTube Chlorine Element Oxidation State The oxidation state of fluorine in chemical compounds is. chlorine is the only element to have changed oxidation state. enter the formula of a chemical compound to find the oxidation number of each element. However, its transition is more complicated than previously. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and. Chlorine Element Oxidation State.
From www.essentialchemicalindustry.org
Chlorine Chlorine Element Oxidation State aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. A net ionic charge can be specified at the. However, its transition is more complicated than previously. . Chlorine Element Oxidation State.
From www.numerade.com
SOLVEDThe oxidation states of the halogens vary from 1 to +7 Chlorine Element Oxidation State chlorine is the only element to have changed oxidation state. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. The oxidation state of an atom is a measure of the degree of oxidation of an atom. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3,. Chlorine Element Oxidation State.
From shiken.ai
Period 3 Oxides Chlorine Element Oxidation State However, its transition is more complicated than previously. chlorine is the only element to have changed oxidation state. The oxidation state of fluorine in chemical compounds is. A net ionic charge can be specified at the. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. The oxidation state of. Chlorine Element Oxidation State.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Element Oxidation State chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is the only element to have changed oxidation state. enter the formula of a chemical compound. Chlorine Element Oxidation State.
From www.youtube.com
Oxidation numbers of chlorine in various compounds and ions YouTube Chlorine Element Oxidation State However, its transition is more complicated than previously. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. A net ionic charge can be specified at the. The oxidation state of fluorine in chemical compounds is. chlorine is a chemical element with atomic number 17 which means there are 17. Chlorine Element Oxidation State.
From www.slideserve.com
PPT Valency & bonding, Oxidation States and redox reaction PowerPoint Chlorine Element Oxidation State the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. The oxidation state of an atom is a measure of the degree of oxidation of an atom. chlorine is the only element to have changed oxidation state. aside from the −1 oxidation states of some. Chlorine Element Oxidation State.
From www.youtube.com
What is the oxidation number of chlorine in Cl2? YouTube Chlorine Element Oxidation State However, its transition is more complicated than previously. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. A net ionic charge can be specified at the. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. The oxidation state of fluorine in. Chlorine Element Oxidation State.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Element Oxidation State However, its transition is more complicated than previously. chlorine is the only element to have changed oxidation state. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. the oxidation state. Chlorine Element Oxidation State.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Chlorine Element Oxidation State the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. enter the formula of a chemical compound to find the oxidation number of each element. The oxidation. Chlorine Element Oxidation State.
From chem.unc.edu
Chloride Oxidation by One or TwoPhoton Excitation of N Chlorine Element Oxidation State chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. The oxidation state of fluorine in chemical compounds is. chlorine is the only element to have changed oxidation state. The oxidation state of an atom is a measure of the degree of oxidation of an atom. aside from the −1. Chlorine Element Oxidation State.
From dreamstime.com
Diagram Representation Of The Element Chlorine Stock Illustration Chlorine Element Oxidation State enter the formula of a chemical compound to find the oxidation number of each element. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. chlorine is the only element to have changed oxidation state. The oxidation state of an atom is a measure of the degree of oxidation. Chlorine Element Oxidation State.
From linatusims.blogspot.com
Oxidation Number of Chlorine LinatuSims Chlorine Element Oxidation State the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the. chlorine is a chemical element with atomic number 17 which means. Chlorine Element Oxidation State.
From slidetodoc.com
Oxidation States of Nitrogen NH 3 HNO 3 Chlorine Element Oxidation State A net ionic charge can be specified at the. However, its transition is more complicated than previously. chlorine is the only element to have changed oxidation state. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. The oxidation state of an atom is a measure of the degree of. Chlorine Element Oxidation State.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Element Oxidation State the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. enter the formula of a chemical compound to find the oxidation number of each element. aside from. Chlorine Element Oxidation State.
From www.chegg.com
Solved 1. Chlorine oxidation states. Chlorine exists in 7 Chlorine Element Oxidation State enter the formula of a chemical compound to find the oxidation number of each element. chlorine is the only element to have changed oxidation state. A net ionic charge can be specified at the. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. the oxidation state of a. Chlorine Element Oxidation State.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Chlorine Element Oxidation State A net ionic charge can be specified at the. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. However, its transition is more complicated than previously. chlorine. Chlorine Element Oxidation State.
From www.chemeurope.com
Periodic table of oxidations states Chlorine Element Oxidation State The oxidation state of an atom is a measure of the degree of oxidation of an atom. A net ionic charge can be specified at the. the oxidation state of a monatomic ion is the same as its charge—for example, na + = +1, cl − = −1. The oxidation state of fluorine in chemical compounds is. chlorine. Chlorine Element Oxidation State.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Chlorine Element Oxidation State A net ionic charge can be specified at the. The oxidation state of an atom is a measure of the degree of oxidation of an atom. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. However, its transition is more complicated than previously. chlorine is the only element to have. Chlorine Element Oxidation State.
From www.youtube.com
How to find the Oxidation Number for Cl in HClO3 (Chloric acid) YouTube Chlorine Element Oxidation State The oxidation state of fluorine in chemical compounds is. However, its transition is more complicated than previously. aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states,. chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. the oxidation state of a. Chlorine Element Oxidation State.