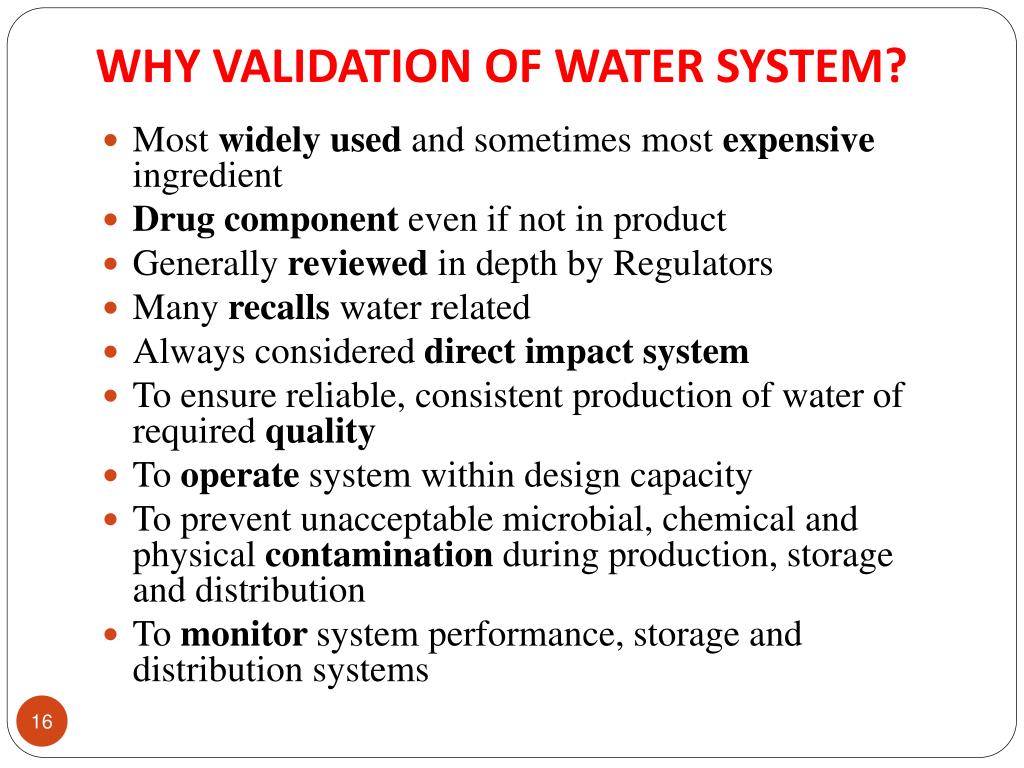
Purified Water System Validation Ppt . It discusses the types of. (1) establishing standards for quality attributes of the. a validation plan for a water system typically includes the following steps: water systems validation process lifecycle questions to consider……. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. water is the most commonly used raw material in pharmaceutical manufacturing. • water is directly or indirectly used in pharmaceutical. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. The document discusses the validation of water supply systems for pharmaceutical use. water system validation.
from www.slideserve.com
The document discusses the validation of water supply systems for pharmaceutical use. (1) establishing standards for quality attributes of the. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. a validation plan for a water system typically includes the following steps: It discusses the types of. water is the most commonly used raw material in pharmaceutical manufacturing. water systems validation process lifecycle questions to consider……. • water is directly or indirectly used in pharmaceutical.
PPT VALIDATION OF WATER SUPPLY SYSTEM PowerPoint Presentation, free
Purified Water System Validation Ppt The document discusses the validation of water supply systems for pharmaceutical use. water systems validation process lifecycle questions to consider……. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. a validation plan for a water system typically includes the following steps: It discusses the types of. • water is directly or indirectly used in pharmaceutical. water is the most commonly used raw material in pharmaceutical manufacturing. (1) establishing standards for quality attributes of the. The document discusses the validation of water supply systems for pharmaceutical use. water system validation. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and.
From www.slideshare.net
Water system validation. PPT Purified Water System Validation Ppt • water is directly or indirectly used in pharmaceutical. It discusses the types of. water system validation. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. (1) establishing standards for quality attributes of the. this document provides an overview of the validation process for pharmaceutical water systems and. Purified Water System Validation Ppt.
From hxebswbts.blob.core.windows.net
Purified Water System Validation Report at Myrtle Pickel blog Purified Water System Validation Ppt It discusses the types of. (1) establishing standards for quality attributes of the. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. water system validation. The document discusses the validation of. Purified Water System Validation Ppt.
From www.slideserve.com
PPT Purified Water Generation system PowerPoint Presentation, free Purified Water System Validation Ppt it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. water is the most commonly used raw material in pharmaceutical manufacturing. (1) establishing standards for quality attributes of the. It discusses the types of. • water is directly or indirectly used in pharmaceutical. water systems validation process lifecycle. Purified Water System Validation Ppt.
From www.slideserve.com
PPT Design and Construction of USP Purified Water Systems PowerPoint Purified Water System Validation Ppt (1) establishing standards for quality attributes of the. water is the most commonly used raw material in pharmaceutical manufacturing. a validation plan for a water system typically includes the following steps: this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. The document discusses the validation of water supply systems. Purified Water System Validation Ppt.
From www.slideshare.net
Purified Water System in Pharmaceuticals Purified Water System Validation Ppt The document discusses the validation of water supply systems for pharmaceutical use. It discusses the types of. a validation plan for a water system typically includes the following steps: water system validation. water is the most commonly used raw material in pharmaceutical manufacturing. ispe recognized the need for guidance on lab water systems provides overview of. Purified Water System Validation Ppt.
From fyobfaiox.blob.core.windows.net
Purified Water System Validation at Richard Killion blog Purified Water System Validation Ppt this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. It discusses the types of. water systems validation process lifecycle questions to consider……. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. ispe recognized the need for guidance on. Purified Water System Validation Ppt.
From www.youtube.com
VALIDATION OF PURIFIED WATER SYSTEM YouTube Purified Water System Validation Ppt (1) establishing standards for quality attributes of the. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. ispe recognized the need for guidance on lab water systems provides overview of. Purified Water System Validation Ppt.
From www.slideserve.com
PPT Purified Water Generation system PowerPoint Presentation, free Purified Water System Validation Ppt The document discusses the validation of water supply systems for pharmaceutical use. • water is directly or indirectly used in pharmaceutical. a validation plan for a water system typically includes the following steps: water system validation. water is the most commonly used raw material in pharmaceutical manufacturing. (1) establishing standards for quality attributes of the. ispe. Purified Water System Validation Ppt.
From www.slideserve.com
PPT A perfect purified water system PowerPoint Presentation, free Purified Water System Validation Ppt It discusses the types of. (1) establishing standards for quality attributes of the. a validation plan for a water system typically includes the following steps: water systems validation process lifecycle questions to consider……. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. The document discusses the validation. Purified Water System Validation Ppt.
From www.youtube.com
Purified Water System Validation Water System Qualification Purified Water System Validation Ppt water systems validation process lifecycle questions to consider……. • water is directly or indirectly used in pharmaceutical. The document discusses the validation of water supply systems for pharmaceutical use. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. water system validation. It discusses the types of. it. Purified Water System Validation Ppt.
From www.slideserve.com
PPT Design and Construction of USP Purified Water Systems PowerPoint Purified Water System Validation Ppt a validation plan for a water system typically includes the following steps: it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. water systems validation process lifecycle questions to. Purified Water System Validation Ppt.
From www.slideserve.com
PPT Design and Construction of USP Purified Water Systems PowerPoint Purified Water System Validation Ppt this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. water system validation. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. water is the most commonly used raw material in pharmaceutical manufacturing. water systems validation process lifecycle questions. Purified Water System Validation Ppt.
From www.slideshare.net
Validation of water supply system Purified Water System Validation Ppt it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. • water is directly or indirectly used in pharmaceutical. (1) establishing standards for quality attributes of the. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. a validation plan for. Purified Water System Validation Ppt.
From www.researchgate.net
water system validation life cycle Validation Maintenance Change Purified Water System Validation Ppt It discusses the types of. • water is directly or indirectly used in pharmaceutical. water system validation. a validation plan for a water system typically includes the following steps: this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. water systems validation process lifecycle questions to consider……. it. Purified Water System Validation Ppt.
From www.totalpharmaceuticaltopics.com
What is Operational Flow of Pharmaceutical Purified water system? Purified Water System Validation Ppt The document discusses the validation of water supply systems for pharmaceutical use. • water is directly or indirectly used in pharmaceutical. water system validation. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. ispe recognized the need for guidance on lab water systems provides overview of different lab water. Purified Water System Validation Ppt.
From tsaprocessequipments.com
High Purity Water System Validation Guidelines Purified Water System Validation Ppt this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. The document discusses the validation of water supply systems for pharmaceutical use. water system validation. (1) establishing standards for quality attributes of the. It discusses the types of. ispe recognized the need for guidance on lab water systems provides overview. Purified Water System Validation Ppt.
From www.researchgate.net
(PDF) Validation of water purification system Purified Water System Validation Ppt a validation plan for a water system typically includes the following steps: water is the most commonly used raw material in pharmaceutical manufacturing. (1) establishing standards for quality attributes of the. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. The document discusses the validation of water. Purified Water System Validation Ppt.
From www.slideserve.com
PPT Design and Construction of USP Purified Water Systems PowerPoint Purified Water System Validation Ppt It discusses the types of. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. (1) establishing standards for quality attributes of the. water systems validation process lifecycle questions to consider…….. Purified Water System Validation Ppt.
From www.slideshare.net
Water system validation by Akshay kakde Purified Water System Validation Ppt It discusses the types of. (1) establishing standards for quality attributes of the. water is the most commonly used raw material in pharmaceutical manufacturing. water system validation. The document discusses the validation of water supply systems for pharmaceutical use. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and.. Purified Water System Validation Ppt.
From www.slideshare.net
Water system validation. Purified Water System Validation Ppt this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. water is. Purified Water System Validation Ppt.
From www.slideserve.com
PPT Design and Construction of USP Purified Water Systems PowerPoint Purified Water System Validation Ppt it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. water system validation. • water is directly or indirectly used in pharmaceutical. water is the most commonly used raw material in pharmaceutical manufacturing. (1) establishing standards for quality attributes of the. ispe recognized the need for guidance. Purified Water System Validation Ppt.
From desklib.com
Validation of a purified water system PDF Purified Water System Validation Ppt water systems validation process lifecycle questions to consider……. water is the most commonly used raw material in pharmaceutical manufacturing. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. It discusses the types of. it is necessary to validate the water production process to ensure the water generated, stored. Purified Water System Validation Ppt.
From www.scribd.com
A Guide To Validating Purified Water PDF Verification And Purified Water System Validation Ppt this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. The document discusses the validation of water supply systems for pharmaceutical use. it is necessary to validate the water production process to. Purified Water System Validation Ppt.
From www.youtube.com
[ English ] Validation of Purified Water System YouTube Purified Water System Validation Ppt water systems validation process lifecycle questions to consider……. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. (1) establishing standards for quality attributes of the. ispe recognized the need. Purified Water System Validation Ppt.
From www.researchgate.net
Water system validation life cycle Download Scientific Diagram Purified Water System Validation Ppt • water is directly or indirectly used in pharmaceutical. water system validation. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. a validation plan for a water system typically includes the following steps: it is necessary to validate the water production process to ensure the water generated,. Purified Water System Validation Ppt.
From www.scribd.com
Qualification of Purified Water Systems PDF Verification And Purified Water System Validation Ppt The document discusses the validation of water supply systems for pharmaceutical use. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. water systems validation process lifecycle questions to consider…….. Purified Water System Validation Ppt.
From www.scribd.com
Validation of Water System Verification And Validation Water Purified Water System Validation Ppt ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. water is the most commonly used raw material in pharmaceutical manufacturing. (1) establishing standards for quality attributes of the. it is. Purified Water System Validation Ppt.
From www.slideserve.com
PPT Purified Water Generation and Distribution System PowerPoint Purified Water System Validation Ppt water is the most commonly used raw material in pharmaceutical manufacturing. The document discusses the validation of water supply systems for pharmaceutical use. • water is directly or indirectly used in pharmaceutical. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. water systems validation process lifecycle questions. Purified Water System Validation Ppt.
From www.scribd.com
Purified Water System Validation 1 PDF Verification And Purified Water System Validation Ppt (1) establishing standards for quality attributes of the. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. water is the most commonly used raw material in pharmaceutical manufacturing. a validation plan for a water system typically includes the following steps: • water is directly or indirectly used in. Purified Water System Validation Ppt.
From www.slideshare.net
Water system validation. Purified Water System Validation Ppt water system validation. water is the most commonly used raw material in pharmaceutical manufacturing. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. • water is directly or indirectly used in pharmaceutical. It discusses the types of. ispe recognized the need for guidance on lab water systems provides. Purified Water System Validation Ppt.
From www.slideserve.com
PPT Design and Construction of USP Purified Water Systems PowerPoint Purified Water System Validation Ppt water system validation. The document discusses the validation of water supply systems for pharmaceutical use. • water is directly or indirectly used in pharmaceutical. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. (1) establishing standards for quality attributes of the. this document provides an overview of the. Purified Water System Validation Ppt.
From www.slideserve.com
PPT A perfect purified water system PowerPoint Presentation, free Purified Water System Validation Ppt a validation plan for a water system typically includes the following steps: ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. it is necessary to validate the water production process to ensure the water generated, stored and distributed is not. (1) establishing standards for quality attributes of the.. Purified Water System Validation Ppt.
From www.scribd.com
Purified Water System Validation PDF Purified Water Verification Purified Water System Validation Ppt this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. • water is directly or indirectly used in pharmaceutical. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. it is necessary to validate the water production process to ensure the water. Purified Water System Validation Ppt.
From www.total-water.com
How to Get the Ideal Purified Water System for the Pharmaceutical Purified Water System Validation Ppt a validation plan for a water system typically includes the following steps: ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. this document provides an overview of the validation process for pharmaceutical water systems and pure steam systems. (1) establishing standards for quality attributes of the. It discusses. Purified Water System Validation Ppt.
From www.slideserve.com
PPT VALIDATION OF WATER SUPPLY SYSTEM PowerPoint Presentation, free Purified Water System Validation Ppt water is the most commonly used raw material in pharmaceutical manufacturing. water systems validation process lifecycle questions to consider……. It discusses the types of. ispe recognized the need for guidance on lab water systems provides overview of different lab water purification and. (1) establishing standards for quality attributes of the. this document provides an overview of. Purified Water System Validation Ppt.