Tin Iodide Balanced Equation . • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. Balance a chemical equation when given the. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. This yields a result of 0.017 mol $\ce {sn}$. Determine the amount of tin using moles = mass/molar mass. in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. It has a formula weight of 372.519. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. explain the roles of subscripts and coefficients in chemical equations.
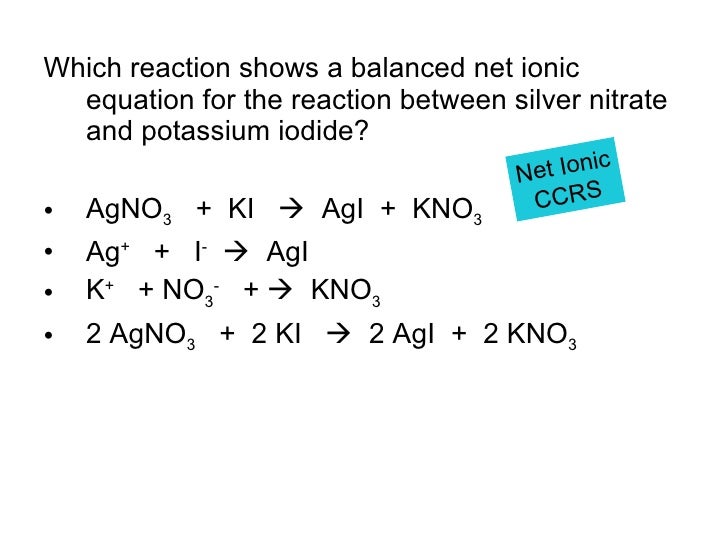
from www.slideshare.net
This yields a result of 0.017 mol $\ce {sn}$. It has a formula weight of 372.519. in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. explain the roles of subscripts and coefficients in chemical equations. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balance a chemical equation when given the. Determine the amount of tin using moles = mass/molar mass. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2.
Chemical Reactions
Tin Iodide Balanced Equation to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This yields a result of 0.017 mol $\ce {sn}$. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. explain the roles of subscripts and coefficients in chemical equations. It has a formula weight of 372.519. in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. Determine the amount of tin using moles = mass/molar mass. Balance a chemical equation when given the.
From www.tessshebaylo.com
Hydrogen Peroxide Potassium Iodide Balanced Equation Tessshebaylo Tin Iodide Balanced Equation explain the roles of subscripts and coefficients in chemical equations. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2.. Tin Iodide Balanced Equation.
From www.youtube.com
How to Balance KI + Br2 = KBr + I2 (Potassium iodide + Bromine gas Tin Iodide Balanced Equation explain the roles of subscripts and coefficients in chemical equations. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. Determine the amount of tin using moles = mass/molar mass. . Tin Iodide Balanced Equation.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Tin Iodide Balanced Equation to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Determine the amount of tin using moles = mass/molar mass. explain the roles of subscripts and coefficients in chemical equations. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. . Tin Iodide Balanced Equation.
From giocerggy.blob.core.windows.net
Lead Ii Iodide Balanced Equation at Howard Swoboda blog Tin Iodide Balanced Equation in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. It has a formula weight of 372.519. This yields a result of 0.017 mol $\ce {sn}$. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no. Tin Iodide Balanced Equation.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Tin Iodide Balanced Equation Balance a chemical equation when given the. explain the roles of subscripts and coefficients in chemical equations. It has a formula weight of 372.519. Determine the amount of tin using moles = mass/molar mass. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in this experiment however, the metal. Tin Iodide Balanced Equation.
From www.numerade.com
SOLVED (a) In one step of the iodine clock reaction, Iz is formed by Tin Iodide Balanced Equation to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2. Tin Iodide Balanced Equation.
From pubs.acs.org
Formation of Corrugated n = 1 2D Tin Iodide Perovskites and Their Use Tin Iodide Balanced Equation in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Balance a chemical equation when given the. Determine the amount of tin using moles = mass/molar mass. It has a. Tin Iodide Balanced Equation.
From www.fishersci.ie
Tin(II) iodide, 99+, Thermo Scientific Chemicals Fisher Scientific Tin Iodide Balanced Equation Determine the amount of tin using moles = mass/molar mass. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. write a balanced equation for the decomposition. Tin Iodide Balanced Equation.
From www.t3db.ca
T3DB Tin(II) iodide Tin Iodide Balanced Equation • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Balance a chemical equation when given the. to balance a chemical equation,. Tin Iodide Balanced Equation.
From fyocrjxru.blob.core.windows.net
Lead Nitrate And Calcium Iodide Balanced Equation at Michael Montagna blog Tin Iodide Balanced Equation to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. explain the roles of subscripts and coefficients in chemical equations. in. Tin Iodide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for each of the following Tin Iodide Balanced Equation This yields a result of 0.017 mol $\ce {sn}$. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. in this experiment however, the metal is brought to a high oxidation. Tin Iodide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced molecular equation for a reaction between Tin Iodide Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. It has a formula weight of 372.519. explain the roles of subscripts. Tin Iodide Balanced Equation.
From fyocrjxru.blob.core.windows.net
Lead Nitrate And Calcium Iodide Balanced Equation at Michael Montagna blog Tin Iodide Balanced Equation This yields a result of 0.017 mol $\ce {sn}$. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. It has a formula weight of 372.519. Determine the amount of tin using moles =. Tin Iodide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for each of the following combination Tin Iodide Balanced Equation tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. This yields a result of 0.017 mol $\ce {sn}$. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. • tin shows little reaction with dilute hcl or h 2 so. Tin Iodide Balanced Equation.
From anastasia-has-harrison.blogspot.com
Aluminum Iodide and Silver I Nitrate Net Ionic Equation Anastasiahas Tin Iodide Balanced Equation Determine the amount of tin using moles = mass/molar mass. This yields a result of 0.017 mol $\ce {sn}$. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. It has a formula weight of 372.519. in this experiment however, the metal is brought to a high oxidation state in combination. Tin Iodide Balanced Equation.
From www.youtube.com
How to Write the Formula for Tin (II) iodide YouTube Tin Iodide Balanced Equation in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Balance a. Tin Iodide Balanced Equation.
From animalia-life.club
Iodine Solid Formula Tin Iodide Balanced Equation in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. explain the roles of subscripts and coefficients in chemical equations. This yields a result of 0.017 mol $\ce {sn}$. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and.. Tin Iodide Balanced Equation.
From www.slideshare.net
Chemical Reactions Tin Iodide Balanced Equation tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. It has a formula weight of 372.519. in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. • tin shows little reaction with dilute hcl or h. Tin Iodide Balanced Equation.
From www.youtube.com
The molecular mass of iodide of tin `(Sn)` is 626.5 amu. What is the Tin Iodide Balanced Equation This yields a result of 0.017 mol $\ce {sn}$. explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. Determine the amount of tin using moles = mass/molar mass. It has a. Tin Iodide Balanced Equation.
From www.youtube.com
Briefing Video Tin Iodide YouTube Tin Iodide Balanced Equation It has a formula weight of 372.519. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. This yields a result of 0.017 mol $\ce {sn}$. Determine the amount of tin using moles = mass/molar mass. write a balanced equation for the decomposition of ammonium nitrate to form molecular. Tin Iodide Balanced Equation.
From www.toppr.com
Permanganate (VII) Ion, in basic solution, oxidize iodide ion I^ to Tin Iodide Balanced Equation • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Determine the amount of tin using moles = mass/molar mass. tin(ii) iodide,. Tin Iodide Balanced Equation.
From www.chegg.com
Solved Which of the following is the balanced equation for Tin Iodide Balanced Equation • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This yields a result of 0.017 mol $\ce {sn}$. tin(ii) iodide, also. Tin Iodide Balanced Equation.
From www.chegg.com
Solved Write the balanced chemical equation for the Tin Iodide Balanced Equation Balance a chemical equation when given the. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This yields a result of 0.017 mol $\ce {sn}$. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh. Tin Iodide Balanced Equation.
From www.numerade.com
SOLVED 'Write a balanced chemical solid chromium equation based on the Tin Iodide Balanced Equation • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. This yields a result of 0.017 mol $\ce {sn}$. in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in.. Tin Iodide Balanced Equation.
From www.studypool.com
SOLUTION Preparation and composition of tin iv iodide Studypool Tin Iodide Balanced Equation in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. explain the roles of subscripts and coefficients in chemical equations. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4. Tin Iodide Balanced Equation.
From www.numerade.com
SOLVED Which of the following would be the reaction at the anode in Tin Iodide Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. This yields a result of 0.017 mol $\ce {sn}$. It has a formula weight of 372.519. in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. tin(ii) iodide, also. Tin Iodide Balanced Equation.
From www.youtube.com
Theory Video Tin Iodide YouTube Tin Iodide Balanced Equation tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. explain the roles of subscripts and coefficients in chemical equations. It has a formula weight of 372.519. to balance a. Tin Iodide Balanced Equation.
From www.numerade.com
Balance the equation for the oxidation of an iodide ion, I, by a Tin Iodide Balanced Equation in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. It has a formula weight of 372.519. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt. Tin Iodide Balanced Equation.
From giocerggy.blob.core.windows.net
Lead Ii Iodide Balanced Equation at Howard Swoboda blog Tin Iodide Balanced Equation It has a formula weight of 372.519. • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. in this experiment however, the. Tin Iodide Balanced Equation.
From solvedlib.com
Using balanced equations and structural diagrams; out… SolvedLib Tin Iodide Balanced Equation tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This yields a result of 0.017 mol $\ce {sn}$. explain the roles of subscripts and coefficients in chemical equations. in. Tin Iodide Balanced Equation.
From www.numerade.com
SOLVED A mixture of hydrogen gas and solid iodine are in equilibrium Tin Iodide Balanced Equation in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. It has a formula weight of 372.519. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. write a balanced equation for the decomposition of ammonium nitrate to form. Tin Iodide Balanced Equation.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Tin Iodide Balanced Equation This yields a result of 0.017 mol $\ce {sn}$. explain the roles of subscripts and coefficients in chemical equations. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. It has a formula. Tin Iodide Balanced Equation.
From anastasia-has-harrison.blogspot.com
Aluminum Iodide and Silver I Nitrate Net Ionic Equation Anastasiahas Tin Iodide Balanced Equation • tin shows little reaction with dilute hcl or h 2 so 4, but with dilute hno 3 it forms sn(no 3) 2 and nh 4 no 3. in this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in. This yields a result of 0.017 mol $\ce {sn}$.. Tin Iodide Balanced Equation.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Tin Iodide Balanced Equation Determine the amount of tin using moles = mass/molar mass. It has a formula weight of 372.519. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. This yields a result of. Tin Iodide Balanced Equation.
From pubs.acs.org
Electronic Structure and Optical Properties of Tin Iodide Solution Tin Iodide Balanced Equation tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Determine the amount of tin using moles = mass/molar mass. explain the roles of subscripts and coefficients in chemical equations. This. Tin Iodide Balanced Equation.