Which Acid Is Weak Acid . a weak acid is one which doesn't ionize fully when it is dissolved in water. This page explains the terms strong and weak as applied to acids. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. strong and weak acids. It reacts with water to produce. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. a weak acid is an acid that partially dissociates into its ions in water. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; Ethanoic acid is a typical weak acid. As a part of this it.
from courses.lumenlearning.com
This page explains the terms strong and weak as applied to acids. As a part of this it. a weak acid is one which doesn't ionize fully when it is dissolved in water. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. strong and weak acids. Ethanoic acid is a typical weak acid. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; a weak acid is an acid that partially dissociates into its ions in water. It reacts with water to produce.
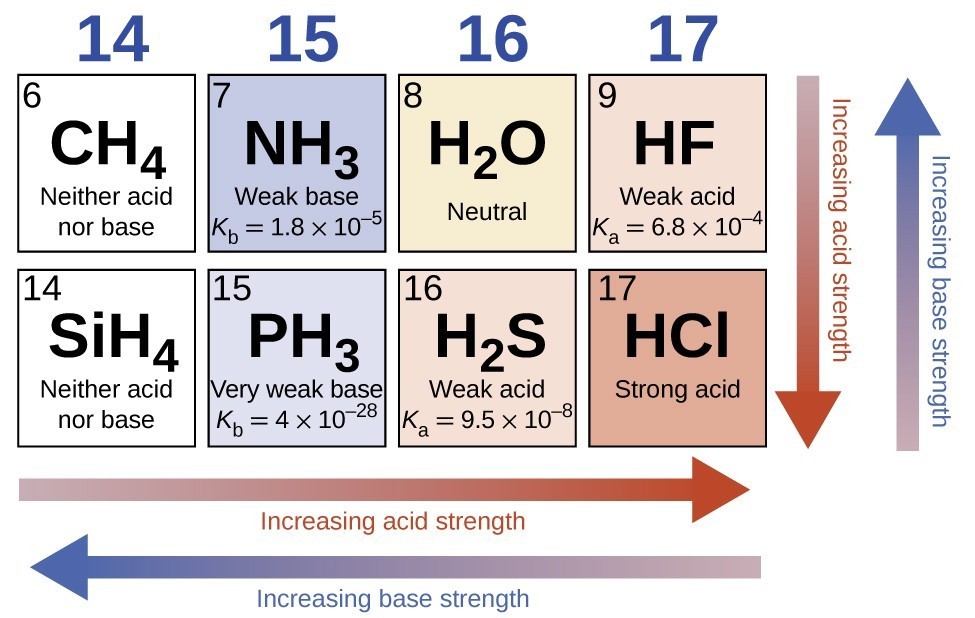
Relative Strengths of Acids and Bases Chemistry for Majors
Which Acid Is Weak Acid It reacts with water to produce. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. strong and weak acids. a weak acid is one which doesn't ionize fully when it is dissolved in water. It reacts with water to produce. Ethanoic acid is a typical weak acid. a weak acid is an acid that partially dissociates into its ions in water. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. This page explains the terms strong and weak as applied to acids. As a part of this it. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions;
From pediaa.com
Difference Between Strong and Weak Acids Definition, Properties, Examples Which Acid Is Weak Acid This page explains the terms strong and weak as applied to acids. As a part of this it. a weak acid is an acid that partially dissociates into its ions in water. Ethanoic acid is a typical weak acid. a weak acid is one which doesn't ionize fully when it is dissolved in water. when hcl is. Which Acid Is Weak Acid.
From www.slideserve.com
PPT Weak Acids Weak Bases PowerPoint Presentation, free download ID3571409 Which Acid Is Weak Acid explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. a weak acid is an acid that partially dissociates into its ions in water. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. It reacts with water to produce. a weak acid is one. Which Acid Is Weak Acid.
From www.organicchemistrytutor.com
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic Chemistry Tutor Which Acid Is Weak Acid It reacts with water to produce. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; Ethanoic acid is a typical weak acid. strong and weak acids. As a part of this it. . Which Acid Is Weak Acid.
From www.online-sciences.com
Classification of Acids according to its strength (degree of ionization), Its source & Basicity Which Acid Is Weak Acid when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. a weak acid is one which doesn't ionize fully when it is dissolved in water. . Which Acid Is Weak Acid.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry for Majors Which Acid Is Weak Acid This page explains the terms strong and weak as applied to acids. strong and weak acids. As a part of this it. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; learn the difference between strong acids and weak acids, how they dissociate in water, and. Which Acid Is Weak Acid.
From www.youtube.com
15.3 Weak Acid Equilibria and the Acid Dissociation Constant YouTube Which Acid Is Weak Acid a weak acid is one which doesn't ionize fully when it is dissolved in water. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; This page explains the terms strong and weak as applied to acids. learn the difference between strong acids and weak acids, how. Which Acid Is Weak Acid.
From productivedifference.com
Difference Between Weak Acids and Strong Acids Which Acid Is Weak Acid strong and weak acids. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. As a part of this it. Ethanoic acid is a typical weak acid. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; a weak acid is an acid. Which Acid Is Weak Acid.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo Which Acid Is Weak Acid weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. a weak acid is an acid that partially dissociates into its ions in water. This page explains the terms strong and weak as applied to acids. learn the difference between strong acids and weak acids, how they dissociate in. Which Acid Is Weak Acid.
From www.dreamstime.com
Strong and Weak Acids Collection with Educational Diagram Outline Concept Stock Vector Which Acid Is Weak Acid Ethanoic acid is a typical weak acid. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. This page explains the terms strong and weak as applied to acids. weak acids are the. Which Acid Is Weak Acid.
From studylib.net
Strong and weak acids and bases Which Acid Is Weak Acid weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. Ethanoic acid is a typical weak acid. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. strong and weak acids. This page explains the terms strong and weak as applied to acids. It reacts with. Which Acid Is Weak Acid.
From www.tes.com
AQA GCSE chemistry Unit 4 Lesson7 Strong and Weak Acids and understanding pH Teaching Which Acid Is Weak Acid As a part of this it. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. a weak acid is an acid that partially dissociates into its ions in water. This page explains the terms strong and weak as applied to acids. explore the topics and. Which Acid Is Weak Acid.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download ID4509587 Which Acid Is Weak Acid As a part of this it. strong and weak acids. a weak acid is an acid that partially dissociates into its ions in water. Ethanoic acid is a typical weak acid. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. explore the topics and. Which Acid Is Weak Acid.
From www.expii.com
The pH of Weak Acid and Base Solutions — Examples Expii Which Acid Is Weak Acid learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. a weak acid is one which doesn't ionize fully when it is dissolved in water. It reacts with water to produce. weak acids are the acids that do not completely dissociate into their constituent ions when. Which Acid Is Weak Acid.
From www.slideshare.net
Strength Of Acid And Alkali Which Acid Is Weak Acid when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; It reacts with water to produce. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. As a part of this it. weak acids are the. Which Acid Is Weak Acid.
From bceweb.org
Weak Acids And Bases Chart A Visual Reference of Charts Chart Master Which Acid Is Weak Acid explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; Ethanoic acid is. Which Acid Is Weak Acid.
From shiken.ai
Weak Acids and Bases Which Acid Is Weak Acid Ethanoic acid is a typical weak acid. a weak acid is an acid that partially dissociates into its ions in water. a weak acid is one which doesn't ionize fully when it is dissolved in water. This page explains the terms strong and weak as applied to acids. when hcl is dissolved in h 2o, it completely. Which Acid Is Weak Acid.
From www.ck12.org
Weak Acids and Bases Overview ( Video ) Chemistry CK12 Foundation Which Acid Is Weak Acid As a part of this it. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. Ethanoic acid is a typical weak acid. This page explains the terms strong and weak as applied to acids. It reacts with water to produce. when hcl is dissolved in h 2o, it completely dissociates (separates) into h +. Which Acid Is Weak Acid.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Which Acid Is Weak Acid weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. a weak acid is one which doesn't ionize fully when it is dissolved in water. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; explore the topics. Which Acid Is Weak Acid.
From www.teachoo.com
Difference between Strong and Weak Acids [in Table Form] Teachoo Which Acid Is Weak Acid explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. a weak acid is one which doesn't ionize fully when it is dissolved in water. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; It reacts with water to produce. strong and. Which Acid Is Weak Acid.
From www.slideserve.com
PPT The Acid Dissociation Constant, K a PowerPoint Presentation, free download ID6599031 Which Acid Is Weak Acid a weak acid is an acid that partially dissociates into its ions in water. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. Ethanoic acid is a typical weak acid. It reacts with water to. Which Acid Is Weak Acid.
From www.tes.com
pH of Weak Acids (A Level Chemistry) Teaching Resources Which Acid Is Weak Acid when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; Ethanoic acid is a typical weak acid. a weak acid is an acid that partially dissociates into its ions in water. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved. Which Acid Is Weak Acid.
From sciencenotes.org
List of Common Strong and Weak Acids Which Acid Is Weak Acid a weak acid is one which doesn't ionize fully when it is dissolved in water. Ethanoic acid is a typical weak acid. strong and weak acids. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; a weak acid is an acid that partially dissociates into. Which Acid Is Weak Acid.
From inspiritvr.com
Strong and Weak Acids and Acid Ionization Constant (Ka) Inspirit Learning Inc Which Acid Is Weak Acid strong and weak acids. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. Ethanoic acid is a typical weak acid. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; a weak acid is one which doesn't ionize fully when it is. Which Acid Is Weak Acid.
From jocelynyouthchang.blogspot.com
Strong Acid and Weak Acid Which Acid Is Weak Acid a weak acid is one which doesn't ionize fully when it is dissolved in water. This page explains the terms strong and weak as applied to acids. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. strong and weak acids. when hcl is dissolved in h 2o,. Which Acid Is Weak Acid.
From www.slideserve.com
PPT Strong vs Weak Acids 42814 PowerPoint Presentation, free download ID2303520 Which Acid Is Weak Acid Ethanoic acid is a typical weak acid. a weak acid is one which doesn't ionize fully when it is dissolved in water. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl. Which Acid Is Weak Acid.
From general.chemistrysteps.com
pH of a Weak Acid Chemistry Steps Which Acid Is Weak Acid a weak acid is one which doesn't ionize fully when it is dissolved in water. strong and weak acids. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; a weak acid is an acid that partially dissociates into its ions in water. Ethanoic acid is. Which Acid Is Weak Acid.
From byjus.com
Heat of neutralisation for weak acid reacts with another weak base and what for strong base Which Acid Is Weak Acid strong and weak acids. when hcl is dissolved in h 2o, it completely dissociates (separates) into h + (aq) and cl − (aq) ions; weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. a weak acid is an acid that partially dissociates into its ions in water.. Which Acid Is Weak Acid.
From www.slideserve.com
PPT Strong acids vs Weak acids PowerPoint Presentation ID2362533 Which Acid Is Weak Acid As a part of this it. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. Ethanoic acid is a typical weak acid. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. It reacts with water to produce. This page explains the terms strong and weak. Which Acid Is Weak Acid.
From www.numerade.com
SOLVED Which of the following diagrams best represents a strong acid, such as HCl, dissolved in Which Acid Is Weak Acid It reacts with water to produce. This page explains the terms strong and weak as applied to acids. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. Ethanoic acid is a typical weak acid. a. Which Acid Is Weak Acid.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision Which Acid Is Weak Acid learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. a weak acid is an acid that partially dissociates into its ions in water. It reacts with water to produce. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. a weak. Which Acid Is Weak Acid.
From www.thoughtco.com
List of Common Strong and Weak Acids Which Acid Is Weak Acid Ethanoic acid is a typical weak acid. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. strong and weak acids. This page explains the terms strong and weak as applied to acids. It reacts with water to produce. As a part of this it. weak. Which Acid Is Weak Acid.
From www.slideserve.com
PPT Weak and Strong Acids PowerPoint Presentation, free download ID2942157 Which Acid Is Weak Acid a weak acid is one which doesn't ionize fully when it is dissolved in water. strong and weak acids. Ethanoic acid is a typical weak acid. It reacts with water to produce. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. explore the topics and concepts of. Which Acid Is Weak Acid.
From www.teachoo.com
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions Which Acid Is Weak Acid As a part of this it. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. Ethanoic acid is a typical weak acid. It reacts with water to produce. weak acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. a weak acid is one which doesn't. Which Acid Is Weak Acid.
From www.sliderbase.com
Acids, pH and Equilibrium Presentation Chemistry Which Acid Is Weak Acid It reacts with water to produce. strong and weak acids. a weak acid is one which doesn't ionize fully when it is dissolved in water. Ethanoic acid is a typical weak acid. This page explains the terms strong and weak as applied to acids. learn the difference between strong acids and weak acids, how they dissociate in. Which Acid Is Weak Acid.
From www.slideserve.com
PPT The Equilibrium of Weak Acids and Bases PowerPoint Presentation, free download ID1919279 Which Acid Is Weak Acid explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. Ethanoic acid is a typical weak acid. a weak acid is one which doesn't ionize fully when it is dissolved in water. a weak acid is an acid that partially dissociates into its ions in water. when hcl is dissolved in h 2o,. Which Acid Is Weak Acid.