From byjus.com
What is the unit of rate constant for first order reaction The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. T 1 / 2 = 69.3 minutes. In other words, if the. ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From dxotqwilz.blob.core.windows.net
Formula Of Rate Constant For First Order Reaction at John Kingston blog The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = ln 2 k. In other words, if the. T 1 / 2 = 69.3 minutes. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. In other words, if the. ln[a] = − kt + ln[a]o. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
first order reaction rate calculation example YouTube The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. In other words, if the. The Rate Constant For 1St Order Reaction Is 69.3.
From askfilo.com
Units of rate constant for a first order reaction. Filo The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. In other words, if the. ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
Units of rate constant calculate rate constant units units of first The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
First Order Reactions Chemistry Class 12 IIT JEE Main + Advanced The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. In other words, if the. T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From www.tessshebaylo.com
Derive An Integrated Rate Equation For Constant The First Order The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. In other words, if the. The Rate Constant For 1St Order Reaction Is 69.3.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. In other words, if the. ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions The Rate Constant For 1St Order Reaction Is 69.3 In other words, if the. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. The Rate Constant For 1St Order Reaction Is 69.3.
From studylib.net
A first order reaction has a rate constant of 1 The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. ln[a] = − kt + ln[a]o. In other words, if the. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
How to calculate the order of a reaction and the rate constant YouTube The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. The Rate Constant For 1St Order Reaction Is 69.3.
From dxoydbizq.blob.core.windows.net
Rate Constant For Zero Order Reaction Is at Esther Rodriguez blog The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o. In other words, if the. T 1 / 2 = 69.3 minutes. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From general.chemistrysteps.com
Rate Law and Reaction Order Chemistry Steps The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. In other words, if the. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From chem.libretexts.org
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. In other words, if the. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
The rate constant for a first order reaction six times when the The Rate Constant For 1St Order Reaction Is 69.3 In other words, if the. ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From askfilo.com
Rate constant k for a first order reaction has been found to be 2.54×10−3.. The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. In other words, if the. The Rate Constant For 1St Order Reaction Is 69.3.
From 9to5science.com
[Solved] Formula for rate constant for the first order 9to5Science The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. ln[a] = − kt + ln[a]o. T 1 / 2 = ln 2 k. In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
The rate constant of a first order reaction is `3 xx 10^(6)" per sec The Rate Constant For 1St Order Reaction Is 69.3 In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. In other words, if the. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From www.researchgate.net
Firstorder reaction rate constants (k obs ) and initial reaction rates The Rate Constant For 1St Order Reaction Is 69.3 In other words, if the. ln[a] = − kt + ln[a]o. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. The Rate Constant For 1St Order Reaction Is 69.3.
From chemistnotes.com
First Order Reaction definition, example, half life period Chemistry The Rate Constant For 1St Order Reaction Is 69.3 In other words, if the. T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
Quickvideo Calculating rate constant from a firstorder reaction The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From askfilo.com
For a first order reaction A→B, the rate constant, k=5.5×10−14 s−1. The t.. The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. In other words, if the. T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From www.meritnation.com
Rate constant of a first order reaction is 2 3*10^4 s^ calculate the The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. ln[a] = − kt + ln[a]o. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. The Rate Constant For 1St Order Reaction Is 69.3.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders The Rate Constant For 1St Order Reaction Is 69.3 In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. T 1 / 2 = 69.3 minutes. ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From www.tessshebaylo.com
Derive Integrated Rate Equation For Constant A First Order Reaction The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. T 1 / 2 = 69.3 minutes. The Rate Constant For 1St Order Reaction Is 69.3.
From www.toppr.com
Derive an integrated rate equation for rate constant of a zero order The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. In other words, if the. T 1 / 2 = 69.3 minutes. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From chemguru.sg
Rate Equation and Order of Reaction The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. The Rate Constant For 1St Order Reaction Is 69.3.
From www.brainkart.com
Rate equation for first order reactions The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. In other words, if the. T 1 / 2 = ln 2 k. T 1 / 2 = 69.3 minutes. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From chem.libretexts.org
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From brainly.in
A first order reaction is 25 complete in 40 minutes calculate the The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From www.slideserve.com
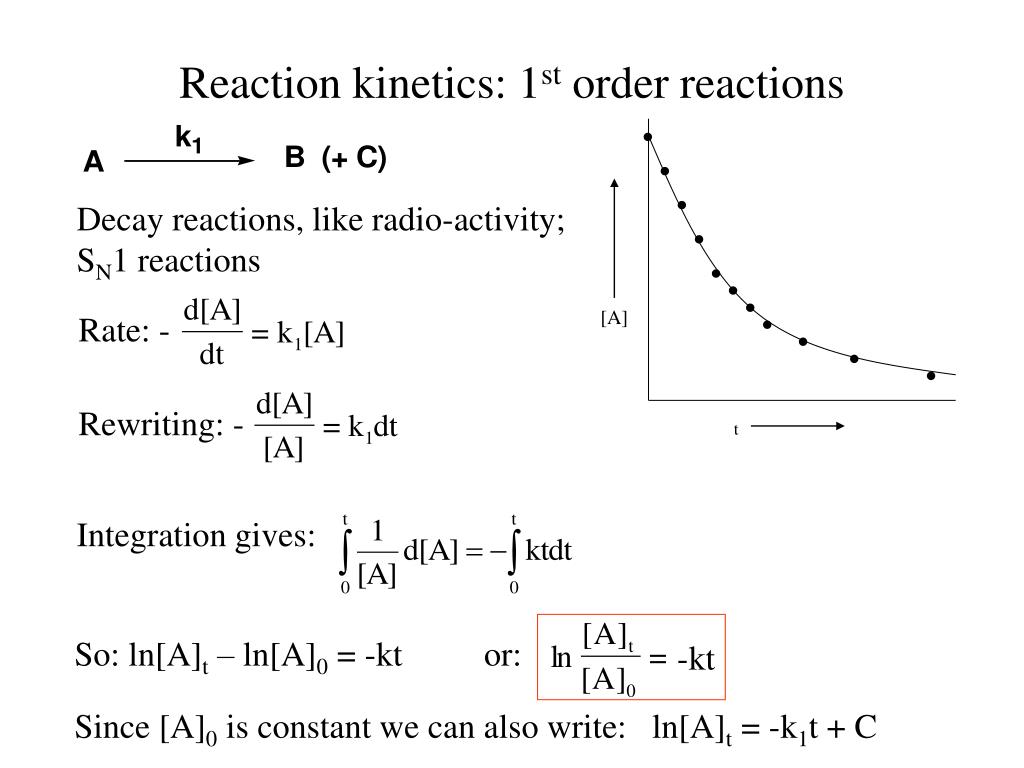
PPT Reaction 1 st order reactions PowerPoint Presentation The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. In other words, if the. ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. In other words, if the. ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. The Rate Constant For 1St Order Reaction Is 69.3.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. The Rate Constant For 1St Order Reaction Is 69.3.