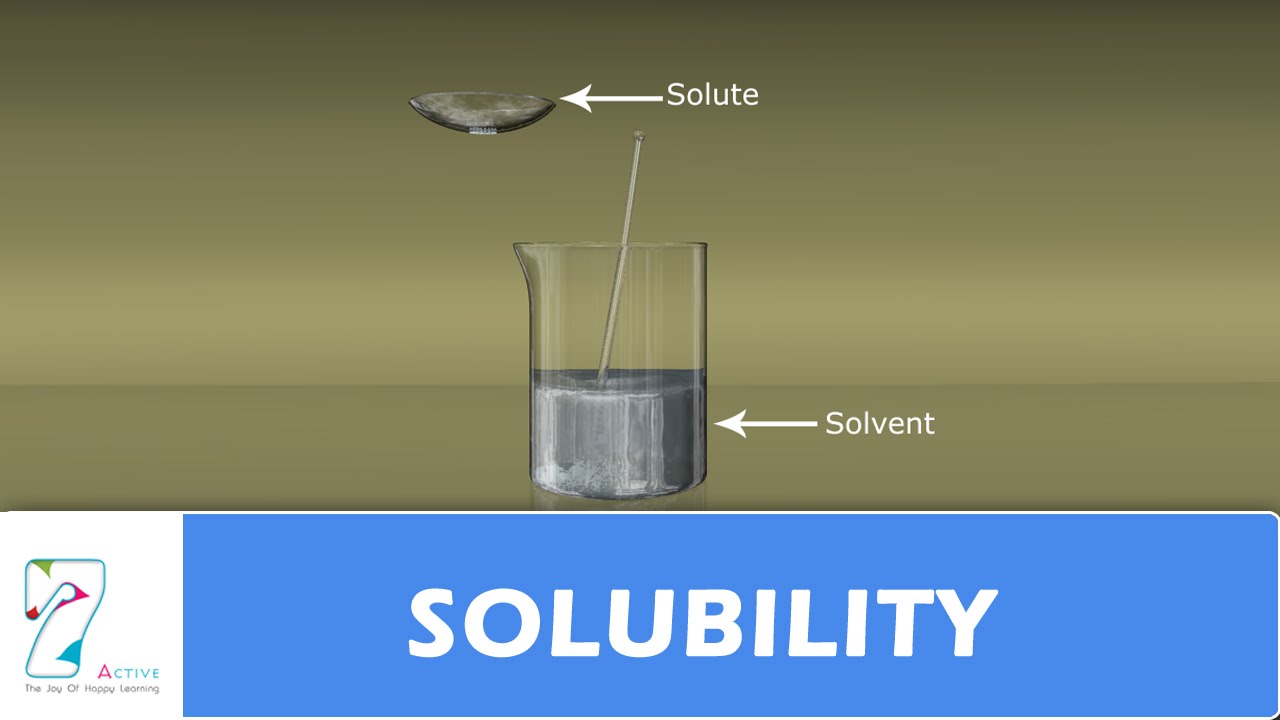
Solubility In Solids . For example, sugar is soluble in water because it dissolves. In such an equilibrium, le. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The constituent particles in a solid. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. If a substance is soluble close solubleable to dissolve in solvent. The process through which a solute in its solid, liquid, or. The solubility of other substances in solids are usually small. Our discussion of solubility in terms of microscopic structure concludes with one more point. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by.
from www.youtube.com
Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. For example, sugar is soluble in water because it dissolves. The process through which a solute in its solid, liquid, or. The solubility of other substances in solids are usually small. The constituent particles in a solid. Our discussion of solubility in terms of microscopic structure concludes with one more point. If a substance is soluble close solubleable to dissolve in solvent. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by.
SOLUBILITY YouTube
Solubility In Solids The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. In such an equilibrium, le. The process through which a solute in its solid, liquid, or. The solubility of other substances in solids are usually small. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. If a substance is soluble close solubleable to dissolve in solvent. The constituent particles in a solid. Our discussion of solubility in terms of microscopic structure concludes with one more point. For example, sugar is soluble in water because it dissolves. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by.
From ar.inspiredpencil.com
Solubility Science Solubility In Solids The solubility of other substances in solids are usually small. The constituent particles in a solid. The process through which a solute in its solid, liquid, or. In such an equilibrium, le. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. If a substance is soluble close. Solubility In Solids.
From ecurrencythailand.com
What Is The Effect Of Temperature On The Solubility Of A Solid In A Solubility In Solids Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. The constituent particles in a solid. The process through which a solute in its solid, liquid, or. In such an equilibrium, le. Our discussion of solubility in terms of microscopic structure concludes with one more point. The solubility of. Solubility In Solids.
From www.docsity.com
Solubility General Chemistry Lecture Slides Docsity Solubility In Solids The process through which a solute in its solid, liquid, or. In such an equilibrium, le. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. If. Solubility In Solids.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.1.4 Solubility翰林国际教育 Solubility In Solids If a substance is soluble close solubleable to dissolve in solvent. In such an equilibrium, le. The solubility of other substances in solids are usually small. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The solubility of a solute in a particular solvent is the maximum. Solubility In Solids.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID6260427 Solubility In Solids In such an equilibrium, le. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by. For example, sugar is soluble in water because it dissolves. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under. Solubility In Solids.
From www.tec-science.com
Alloys complete solubility of components in solid state (solid Solubility In Solids The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. For example, sugar is soluble in water because it dissolves. In such an equilibrium, le. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature.. Solubility In Solids.
From www.slideserve.com
PPT Solubility PowerPoint Presentation ID5581895 Solubility In Solids The solubility of other substances in solids are usually small. If a substance is soluble close solubleable to dissolve in solvent. For example, sugar is soluble in water because it dissolves. In such an equilibrium, le. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The solubility. Solubility In Solids.
From www.youtube.com
Solubility of Solids at Different Temperatures Experiment YouTube Solubility In Solids Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. Our discussion of solubility in terms of microscopic structure concludes with one more point. In such an. Solubility In Solids.
From chem.libretexts.org
13.2 Solubility and Molecular Structure Chemistry LibreTexts Solubility In Solids The constituent particles in a solid. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by. The solubility of other substances in solids are usually small. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved. Solubility In Solids.
From socratic.org
How would you determine the solubility of potassium nitrate? Socratic Solubility In Solids The constituent particles in a solid. Our discussion of solubility in terms of microscopic structure concludes with one more point. The process through which a solute in its solid, liquid, or. The solubility of other substances in solids are usually small. If a substance is soluble close solubleable to dissolve in solvent. The solubility of a solid or a liquid. Solubility In Solids.
From media.ed.science.psu.edu
Solubility of ionic solids as a function of temperature Solubility In Solids Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The constituent particles in a solid. The solubility of other substances in solids are usually small. For example, sugar is soluble in water because it dissolves. Our discussion of solubility in terms of microscopic structure concludes with one. Solubility In Solids.
From www.youtube.com
SOLUBILITY YouTube Solubility In Solids Our discussion of solubility in terms of microscopic structure concludes with one more point. If a substance is soluble close solubleable to dissolve in solvent. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. Solubility is the maximum concentration of a solute that can dissolve in a specific. Solubility In Solids.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Solubility In Solids The constituent particles in a solid. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. Solubility is the maximum concentration of a solute that can. Solubility In Solids.
From www.numerade.com
SOLVED Q3. Explain the factors that affect solubility of gases and Solubility In Solids For example, sugar is soluble in water because it dissolves. The constituent particles in a solid. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by. The process through which a solute in its solid, liquid, or. Our discussion of solubility in terms. Solubility In Solids.
From www.vrogue.co
Solved Activity 2 1 Solubility Chart Use The Chart Be vrogue.co Solubility In Solids Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. Our discussion of solubility in terms of microscopic structure concludes with one more point. The process through which a solute in its solid, liquid, or. Solubility is the maximum concentration of a solute that can dissolve in a specific. Solubility In Solids.
From www.breakingatom.com
Solubility of Elements and Compounds Solubility In Solids The solubility of other substances in solids are usually small. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by. If. Solubility In Solids.
From www.shutterstock.com
Solubility Graph Variation Solubility Different Solids Stock Vector Solubility In Solids If a substance is soluble close solubleable to dissolve in solvent. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. The constituent particles in a solid. The solubility of other substances in solids are usually small. In such an equilibrium, le. Our discussion of solubility in terms of. Solubility In Solids.
From chem.libretexts.org
13.3 Factors Affecting Solubility Chemistry LibreTexts Solubility In Solids The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by. The process through which a solute in its solid, liquid, or. The solubility of other substances in solids are usually small. Our discussion of solubility in terms of microscopic structure concludes with one. Solubility In Solids.
From www.dreamstime.com
Dissolving Solids. Solubility Chemistry Stock Vector Illustration of Solubility In Solids Our discussion of solubility in terms of microscopic structure concludes with one more point. The constituent particles in a solid. If a substance is soluble close solubleable to dissolve in solvent. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The solubility of a solute in a. Solubility In Solids.
From www.ck12.org
Solubility CK12 Foundation Solubility In Solids Our discussion of solubility in terms of microscopic structure concludes with one more point. If a substance is soluble close solubleable to dissolve in solvent. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. In such an equilibrium, le. The constituent particles in a solid. The. Solubility In Solids.
From rayb78.github.io
Solubility In Water Chart Solubility In Solids The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by. For example, sugar is soluble in water because it dissolves. In such an equilibrium, le. The solubility of other substances in solids are usually small. The process through which a solute in its. Solubility In Solids.
From www.vedantu.com
What is the relationship between the solubility and the temperature Solubility In Solids In such an equilibrium, le. Our discussion of solubility in terms of microscopic structure concludes with one more point. The process through which a solute in its solid, liquid, or. For example, sugar is soluble in water because it dissolves. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at. Solubility In Solids.
From www.studypool.com
SOLUTION Difference between solubility and dissolution importance of Solubility In Solids The process through which a solute in its solid, liquid, or. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by. The solubility of other substances in solids are usually small. Solubility is the maximum concentration of a solute that can dissolve in. Solubility In Solids.
From www.slideserve.com
PPT Chapter 4 Equilibrium Phase Diagrams and The IronCarbon system Solubility In Solids The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The process through which a solute in its solid, liquid, or.. Solubility In Solids.
From chem.libretexts.org
14.3 Aqueous Solutions of Solids Chemistry LibreTexts Solubility In Solids The constituent particles in a solid. The solubility of other substances in solids are usually small. If a substance is soluble close solubleable to dissolve in solvent. Our discussion of solubility in terms of microscopic structure concludes with one more point. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent. Solubility In Solids.
From courses.lumenlearning.com
Solubility Chemistry Atoms First Solubility In Solids The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. In such an equilibrium, le. The process through which a solute in its solid, liquid, or. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of. Solubility In Solids.
From www.reddit.com
Spontaneous Reactions and solids that will dissolve in acids chemhelp Solubility In Solids The constituent particles in a solid. The solubility of other substances in solids are usually small. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. In such an equilibrium, le. For example, sugar is soluble in water because it dissolves. If a substance is soluble close solubleable. Solubility In Solids.
From www.blendspace.com
Chapter 14 Aqueous Solutions Lessons Blendspace Solubility In Solids Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The constituent particles in a solid. The process through which a solute in its solid, liquid, or. For example, sugar is soluble in water because it dissolves. Solubility is defined as the upper limit of solute that can. Solubility In Solids.
From www.gradegorilla.com
iGCSE Chemistry Solubility Grade Gorilla Solubility In Solids The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. The process through which a solute in its solid, liquid, or. The solubility of other substances in solids are usually small. The constituent particles in a solid. In such an equilibrium, le. Solubility is defined as the. Solubility In Solids.
From www.tec-science.com
Typs of alloys tecscience Solubility In Solids For example, sugar is soluble in water because it dissolves. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of a gaseous solute is affected by.. Solubility In Solids.
From brainly.in
The graph shows the solubility in water of 4 solids at different Solubility In Solids In such an equilibrium, le. The constituent particles in a solid. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. The solubility of other substances in solids are usually small. For example, sugar is soluble in water because it dissolves. The solubility of a solid or. Solubility In Solids.
From ar.inspiredpencil.com
Solubility Examples Solubility In Solids The constituent particles in a solid. The solubility of other substances in solids are usually small. The process through which a solute in its solid, liquid, or. In such an equilibrium, le. If a substance is soluble close solubleable to dissolve in solvent. For example, sugar is soluble in water because it dissolves. Our discussion of solubility in terms of. Solubility In Solids.
From infogram.com
Solubility Infographic Infogram Solubility In Solids If a substance is soluble close solubleable to dissolve in solvent. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. The constituent particles in a. Solubility In Solids.
From www.thoughtco.com
Solubility Definition in Chemistry Solubility In Solids The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. The solubility of other substances in solids are usually small. In such an equilibrium, le. Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. If. Solubility In Solids.
From cartoondealer.com
Solubility Cartoons, Illustrations & Vector Stock Images 155 Pictures Solubility In Solids The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution. The constituent particles in a solid. The solubility of other substances in solids are usually small. The solubility of a solid or a liquid solute in a solvent is affected by the temperature, while the solubility of. Solubility In Solids.