Calorimetry Example . learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. See examples of heat transfer between. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. learn how to determine the specific heat of a metal using a coffee cup calorimeter. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical.
from www.linstitute.net
learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. See examples of heat transfer between. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to determine the specific heat of a metal using a coffee cup calorimeter. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation.
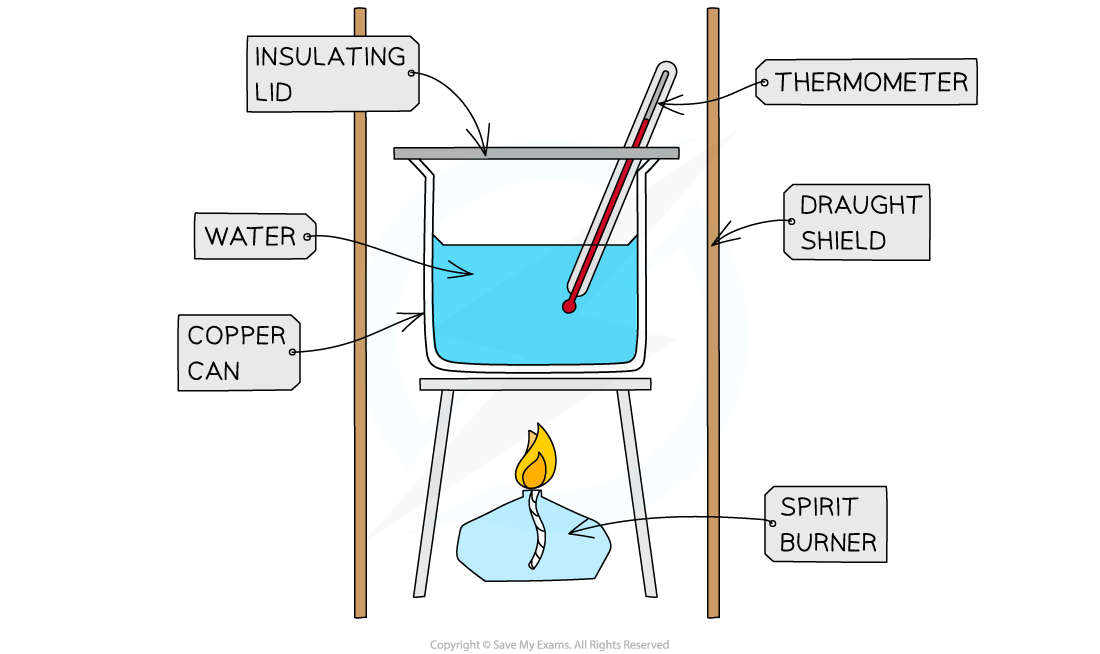
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育
Calorimetry Example learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to determine the specific heat of a metal using a coffee cup calorimeter. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. See examples of heat transfer between.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of. Calorimetry Example.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Example learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. See examples of heat transfer between. learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. learn how to determine the specific heat of a metal using a coffee. Calorimetry Example.
From users.highland.edu
Calorimetry Calorimetry Example learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. learn how to measure the amount of heat. Calorimetry Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. See examples of heat transfer. Calorimetry Example.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Example learn how to determine the specific heat of a metal using a coffee cup calorimeter. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. learn how to use calorimetry to measure. Calorimetry Example.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Example learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. learn how to measure. Calorimetry Example.
From giobxedsi.blob.core.windows.net
Calorimeter Experiment Calculation at Mabel Ballenger blog Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of heat involved. Calorimetry Example.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Example learn how to determine the specific heat of a metal using a coffee cup calorimeter. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. learn how. Calorimetry Example.
From sweetnowsw.blogspot.com
Calorimetry Problems Worksheet Specific Heat And Calorimetry Packet Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. learn how to measure the amount of. Calorimetry Example.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Example learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. See examples of heat transfer between. calorimetry is the measurement of the transfer of heat into or out of a. Calorimetry Example.
From www.slideserve.com
PPT Constant Pressure Calorimetry Another Example PowerPoint Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. See examples of heat transfer between. learn how to determine the specific heat of a metal using a coffee cup calorimeter. learn how to. Calorimetry Example.
From saylordotorg.github.io
Calorimetry Calorimetry Example See examples of heat transfer between. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is. Calorimetry Example.
From www.slideserve.com
PPT 18 Heat and the First Law of Thermodynamics PowerPoint Calorimetry Example learn how to determine the specific heat of a metal using a coffee cup calorimeter. See examples of heat transfer between. learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to. Calorimetry Example.
From www.slideserve.com
PPT Constant Pressure Calorimetry Another Example PowerPoint Calorimetry Example learn how to determine the specific heat of a metal using a coffee cup calorimeter. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. See examples of heat transfer between. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry. Calorimetry Example.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Example learn how to determine the specific heat of a metal using a coffee cup calorimeter. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of heat involved in. Calorimetry Example.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID1598001 Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. See examples of heat transfer between. calorimetry is the measurement of the transfer of heat into or out of a. Calorimetry Example.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry Example See examples of heat transfer between. learn how to determine the specific heat of a metal using a coffee cup calorimeter. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. learn how to measure the amount of heat involved in chemical or physical processes using. Calorimetry Example.
From www.wizeprep.com
Calorimetry Wize University Chemistry Textbook Wizeprep Calorimetry Example learn how to determine the specific heat of a metal using a coffee cup calorimeter. See examples of heat transfer between. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry. Calorimetry Example.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Example learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. in chemistry and thermodynamics, calorimetry (from. Calorimetry Example.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Example learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. See examples of heat transfer between. learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. calorimetry is. Calorimetry Example.
From www.slideserve.com
PPT Reactive chemical hazards PowerPoint Presentation, free download Calorimetry Example See examples of heat transfer between. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry. Calorimetry Example.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Example learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to determine the specific heat of a metal using a coffee cup calorimeter. learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. learn how to measure the amount of heat involved. Calorimetry Example.
From www.scribd.com
calorimetry Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. See examples of heat transfer between. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry. Calorimetry Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimetry Example learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. See examples of heat transfer between. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. . Calorimetry Example.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Example learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn. Calorimetry Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1875569 Calorimetry Example learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. learn how to determine the specific heat of a metal using a coffee cup calorimeter. learn how to measure the amount of heat. Calorimetry Example.
From www.slideserve.com
PPT Section 7.2—Calorimetry & Heat Capacity PowerPoint Presentation Calorimetry Example See examples of heat transfer between. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. . Calorimetry Example.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimetry Example learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. See examples of heat transfer between. learn how to determine the specific heat of a metal using a coffee cup calorimeter. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. in. Calorimetry Example.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. See examples of heat transfer between. learn how to determine the specific heat of a metal using a coffee cup calorimeter. learn. Calorimetry Example.
From www.youtube.com
Calorimetry Example Calculation YouTube Calorimetry Example learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. learn how to determine the specific heat of a metal using a coffee cup calorimeter. See examples of heat transfer between. in chemistry and thermodynamics,. Calorimetry Example.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to determine the specific heat of a metal using a coffee cup calorimeter. See examples of heat transfer between. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. learn. Calorimetry Example.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Example learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called. See examples of heat transfer between. learn how. Calorimetry Example.
From www.slideserve.com
PPT Molar Enthalpy and Calorimetry PowerPoint Presentation, free Calorimetry Example learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. learn how to determine the specific heat of a metal using a coffee cup calorimeter. in chemistry and thermodynamics, calorimetry (from. Calorimetry Example.
From www.slideserve.com
PPT Introduction to Thermochemistry PowerPoint Presentation, free Calorimetry Example See examples of heat transfer between. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical.. Calorimetry Example.
From fyozfywtx.blob.core.windows.net
Bomb Calorimetry Examples at Alex Kowalski blog Calorimetry Example in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. learn how to determine the specific heat of a metal using a coffee cup calorimeter. learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. learn how to measure the amount of heat. Calorimetry Example.