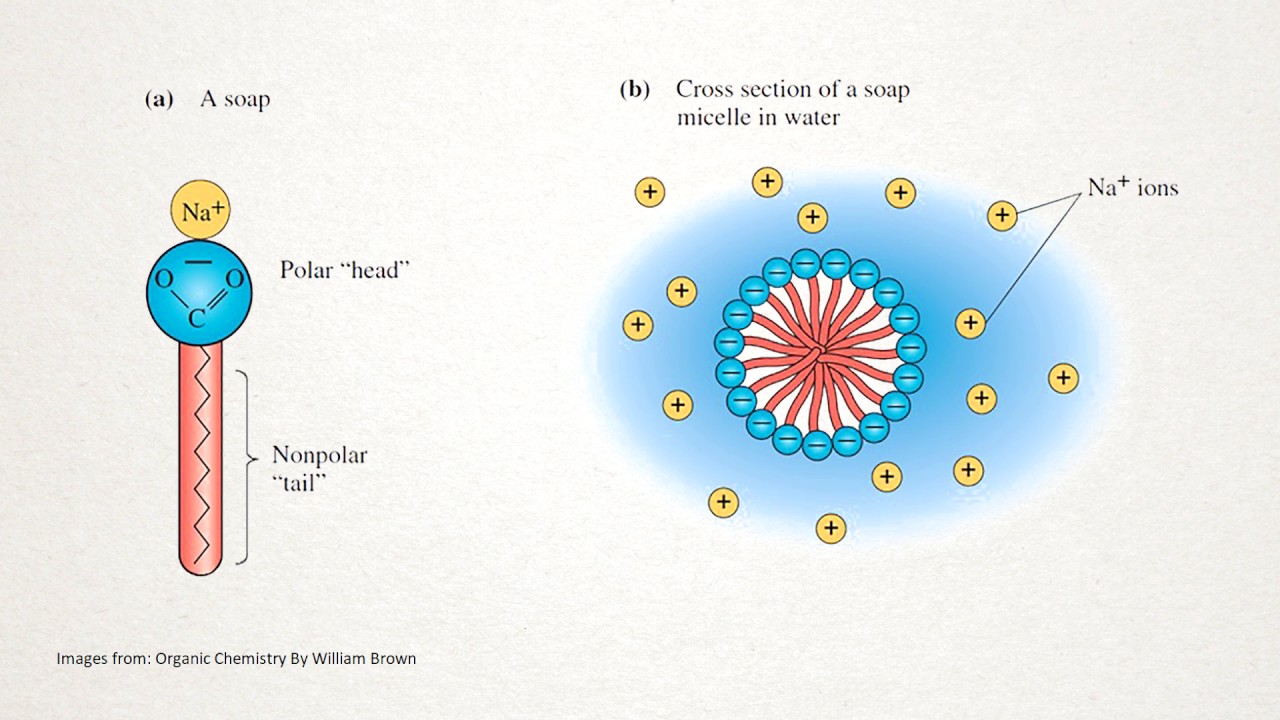
How Does Soap Work Reddit . learn how soap cleans by acting as an emulsifier and dispersing oil and dirt in water. One attracted to water and one repelled by it. Soap is made of a chain, one end loves water, the other end doesn't like water, but this side surrounds oil and gunk. When immersed in water, the. If you pour some oil into water, it will form separate layers. how soap works is due to its unique chemistry, the hydrophilic. learn how soap molecules have two ends: soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. soap is a simple but powerful tool to fight germs and viruses. normally, oils are not soluble in water. learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. Find out the structure, properties, and disadvantages of soap molecules. Learn how it works and why it is so effective in this article from. Soap binds to oils and fats and. Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs.
from www.youtube.com
normally, oils are not soluble in water. soap is a simple but powerful tool to fight germs and viruses. Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. Find out the structure, properties, and disadvantages of soap molecules. One attracted to water and one repelled by it. Learn how it works and why it is so effective in this article from. soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. Soap is made of a chain, one end loves water, the other end doesn't like water, but this side surrounds oil and gunk. how soap works is due to its unique chemistry, the hydrophilic.
How does Soap Work? YouTube
How Does Soap Work Reddit Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. If you pour some oil into water, it will form separate layers. Learn how it works and why it is so effective in this article from. Soap binds to oils and fats and. soap is a simple but powerful tool to fight germs and viruses. Soap is made of a chain, one end loves water, the other end doesn't like water, but this side surrounds oil and gunk. soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. normally, oils are not soluble in water. learn how soap cleans by acting as an emulsifier and dispersing oil and dirt in water. learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. One attracted to water and one repelled by it. Find out the structure, properties, and disadvantages of soap molecules. When immersed in water, the. learn how soap molecules have two ends: how soap works is due to its unique chemistry, the hydrophilic.
From nearnature.co
How Does The Soap Work How Does Soap Work Reddit soap is a simple but powerful tool to fight germs and viruses. One attracted to water and one repelled by it. soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. Soap is made of a chain, one. How Does Soap Work Reddit.
From www.youtube.com
How does soap work? (3D Animation) YouTube How Does Soap Work Reddit Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. Learn how it works and why it is so effective in this article from. normally, oils are not soluble in water. soap is a simple but powerful tool to fight germs and viruses. When immersed in water, the. Find. How Does Soap Work Reddit.
From www.youtube.com
How Does Soap Work? YouTube How Does Soap Work Reddit learn how soap cleans by acting as an emulsifier and dispersing oil and dirt in water. Find out the structure, properties, and disadvantages of soap molecules. Learn how it works and why it is so effective in this article from. how soap works is due to its unique chemistry, the hydrophilic. learn how soap molecules with hydrophilic. How Does Soap Work Reddit.
From www.futurescientist.org
Resources Future Scientist How Does Soap Work Reddit learn how soap molecules have two ends: If you pour some oil into water, it will form separate layers. how soap works is due to its unique chemistry, the hydrophilic. Soap binds to oils and fats and. One attracted to water and one repelled by it. soap is a simple but powerful tool to fight germs and. How Does Soap Work Reddit.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Reddit Soap is made of a chain, one end loves water, the other end doesn't like water, but this side surrounds oil and gunk. how soap works is due to its unique chemistry, the hydrophilic. soap is a simple but powerful tool to fight germs and viruses. Soap binds to oils and fats and. When immersed in water, the.. How Does Soap Work Reddit.
From www.youtube.com
How does Soap Work? YouTube How Does Soap Work Reddit Soap binds to oils and fats and. Find out the structure, properties, and disadvantages of soap molecules. learn how soap molecules have two ends: If you pour some oil into water, it will form separate layers. One attracted to water and one repelled by it. Soap is made of a chain, one end loves water, the other end doesn't. How Does Soap Work Reddit.
From gioqpaezg.blob.core.windows.net
Does Shampoo Kill Germs Like Soap at Valarie Smith blog How Does Soap Work Reddit Find out the structure, properties, and disadvantages of soap molecules. soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. how soap works is due to its unique chemistry, the hydrophilic. Soap binds to oils and fats and. learn how soap cleans by acting as an emulsifier and dispersing oil and dirt in. How Does Soap Work Reddit.
From exyecafzz.blob.core.windows.net
How Does Pump Soap Work at Jason Hunt blog How Does Soap Work Reddit soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. normally, oils are not soluble in water. One attracted to water and one repelled by it. soap is a simple but powerful tool to fight germs and viruses. Soap can clean hands and dishes by forming bridges between water and oily substances, and. How Does Soap Work Reddit.
From www.slideshare.net
How do soaps work How Does Soap Work Reddit If you pour some oil into water, it will form separate layers. normally, oils are not soluble in water. soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. One attracted to water and one repelled by it. Find out the structure, properties, and disadvantages of soap molecules. soap is a simple but. How Does Soap Work Reddit.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Reddit When immersed in water, the. learn how soap molecules have two ends: how soap works is due to its unique chemistry, the hydrophilic. soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. learn how soap cleans by acting as an emulsifier and dispersing oil and dirt in water. Soap binds to. How Does Soap Work Reddit.
From entreasmemorias.blogspot.com
76 HD What Are Hydrophobic And Hydrophilic Parts In Soap insectza How Does Soap Work Reddit how soap works is due to its unique chemistry, the hydrophilic. Soap is made of a chain, one end loves water, the other end doesn't like water, but this side surrounds oil and gunk. learn how soap molecules have two ends: learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. Soap. How Does Soap Work Reddit.
From www.meritech.com
How Does Soap Work? How Soap Works to Remove Germs and Pathogens How Does Soap Work Reddit If you pour some oil into water, it will form separate layers. how soap works is due to its unique chemistry, the hydrophilic. Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. Find out the structure, properties, and disadvantages of soap molecules. One attracted to water and one repelled. How Does Soap Work Reddit.
From www.tffn.net
The Chemistry of Soap How Does it Work? The Enlightened Mindset How Does Soap Work Reddit learn how soap molecules have two ends: soap is a simple but powerful tool to fight germs and viruses. When immersed in water, the. Soap is made of a chain, one end loves water, the other end doesn't like water, but this side surrounds oil and gunk. learn how soap cleans by acting as an emulsifier and. How Does Soap Work Reddit.
From www.theodysseyonline.com
How Does Soap Work? How Does Soap Work Reddit normally, oils are not soluble in water. Soap binds to oils and fats and. Learn how it works and why it is so effective in this article from. learn how soap cleans by acting as an emulsifier and dispersing oil and dirt in water. soap is a simple but powerful tool to fight germs and viruses. . How Does Soap Work Reddit.
From www.freepik.com
Premium Vector How soap works vector illustration infographic How Does Soap Work Reddit learn how soap molecules have two ends: When immersed in water, the. Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. normally, oils are not soluble in water. Find out the structure, properties, and disadvantages of soap molecules. learn how soap molecules with hydrophilic heads and hydrophobic. How Does Soap Work Reddit.
From www.thebodybean.com
How does Soap work? — The Body Bean How Does Soap Work Reddit If you pour some oil into water, it will form separate layers. normally, oils are not soluble in water. how soap works is due to its unique chemistry, the hydrophilic. soap is a simple but powerful tool to fight germs and viruses. learn how soap cleans by acting as an emulsifier and dispersing oil and dirt. How Does Soap Work Reddit.
From health.howstuffworks.com
Does bar soap work better than liquid soap? HowStuffWorks How Does Soap Work Reddit One attracted to water and one repelled by it. Soap is made of a chain, one end loves water, the other end doesn't like water, but this side surrounds oil and gunk. When immersed in water, the. learn how soap cleans by acting as an emulsifier and dispersing oil and dirt in water. soap molecules have a hydrophilic. How Does Soap Work Reddit.
From www.belomed.com
Why Soap Is Effective at Destroying the Coronavirus Belo Medical Group How Does Soap Work Reddit Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. Soap binds to oils and fats and. how soap works is due to its unique chemistry, the hydrophilic. One attracted to water and one. How Does Soap Work Reddit.
From www.youtube.com
How Does Soap Work? YouTube How Does Soap Work Reddit normally, oils are not soluble in water. learn how soap molecules have two ends: soap is a simple but powerful tool to fight germs and viruses. how soap works is due to its unique chemistry, the hydrophilic. When immersed in water, the. Find out the structure, properties, and disadvantages of soap molecules. learn how soap. How Does Soap Work Reddit.
From botpenguin.com
SOAP API (Simple Object Access Protocol) BotPenguin How Does Soap Work Reddit soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. how soap works is due to its unique chemistry, the hydrophilic. If you pour some oil into water, it will form separate layers. Learn how it works and why it is so effective in this article from. One attracted to water and one repelled. How Does Soap Work Reddit.
From youtube.com
How Does Soap Work? YouTube How Does Soap Work Reddit soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. how soap works is due to its unique chemistry, the hydrophilic. learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. learn how soap molecules have two ends: Soap is made of a chain, one end loves. How Does Soap Work Reddit.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Reddit soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. soap is a simple but powerful tool to fight germs and viruses. normally, oils are not soluble in water. One attracted to water and one repelled by it. When immersed in water, the. learn how soap cleans by acting as an emulsifier. How Does Soap Work Reddit.
From guernseydonkey.com
How does soap work? How Does Soap Work Reddit how soap works is due to its unique chemistry, the hydrophilic. If you pour some oil into water, it will form separate layers. learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. normally, oils are not soluble in water. Soap can clean hands and dishes by forming bridges between water and. How Does Soap Work Reddit.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Reddit how soap works is due to its unique chemistry, the hydrophilic. soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. Soap is made of a chain, one end loves water, the other end doesn't. How Does Soap Work Reddit.
From www.meritech.com
How Does Soap Work? How Soap Works to Remove Germs and Pathogens How Does Soap Work Reddit learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. Learn how it works and why it is so effective in this article from. Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. When immersed in water, the. learn how soap cleans by. How Does Soap Work Reddit.
From exyecafzz.blob.core.windows.net
How Does Pump Soap Work at Jason Hunt blog How Does Soap Work Reddit soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. normally, oils are not soluble in water. soap is a simple but powerful tool to fight germs and viruses. Learn how it works and why it is so effective in this article from. Soap binds to oils and fats and. learn how. How Does Soap Work Reddit.
From newtondesk.com
Why Are Bubbles Formed In Soap Solution? Types of Soap How Does Soap Work Reddit When immersed in water, the. soap is a simple but powerful tool to fight germs and viruses. Soap binds to oils and fats and. If you pour some oil into water, it will form separate layers. normally, oils are not soluble in water. One attracted to water and one repelled by it. how soap works is due. How Does Soap Work Reddit.
From www.theodysseyonline.com
How Does Soap Work? How Does Soap Work Reddit Soap binds to oils and fats and. soap is a simple but powerful tool to fight germs and viruses. One attracted to water and one repelled by it. learn how soap cleans by acting as an emulsifier and dispersing oil and dirt in water. Soap is made of a chain, one end loves water, the other end doesn't. How Does Soap Work Reddit.
From atelier-yuwa.ciao.jp
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab Muffin How Does Soap Work Reddit soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. soap is a simple but powerful tool to fight germs and viruses. Soap binds to oils and fats and. Find out the structure, properties, and disadvantages of soap molecules. Soap can clean hands and dishes by forming bridges between water and oily substances, and. How Does Soap Work Reddit.
From ar.inspiredpencil.com
Soap Molecule Structure How Does Soap Work Reddit learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. Learn how it works and why it is so effective in this article from. One attracted to water and one repelled by it. Find out the structure, properties, and disadvantages of soap molecules. soap molecules have a hydrophilic (water loving) head and a. How Does Soap Work Reddit.
From hxeifyvuj.blob.core.windows.net
Soap That Kills Bacteria at James Ricks blog How Does Soap Work Reddit soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. learn how soap molecules have two ends: soap is a simple but powerful tool to fight germs and viruses. Soap binds to oils and fats and. One attracted to water and one repelled by it. learn how soap cleans by acting as. How Does Soap Work Reddit.
From www.youtube.com
How Does Soap Work? YouTube How Does Soap Work Reddit how soap works is due to its unique chemistry, the hydrophilic. Find out the structure, properties, and disadvantages of soap molecules. When immersed in water, the. learn how soap molecules have two ends: Learn how it works and why it is so effective in this article from. learn how soap cleans by acting as an emulsifier and. How Does Soap Work Reddit.
From www.reddit.com
How to find Best Bathing Soap for Face In India for Daily Use for Clean How Does Soap Work Reddit Soap can clean hands and dishes by forming bridges between water and oily substances, and by breaking apart germs. soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. how soap works is due to its unique chemistry, the hydrophilic. Find out the structure, properties, and disadvantages of soap molecules. If you pour some. How Does Soap Work Reddit.
From www.reddit.com
Why SOAP works so well!! r/Images How Does Soap Work Reddit learn how soap cleans by acting as an emulsifier and dispersing oil and dirt in water. Soap binds to oils and fats and. soap molecules have a hydrophilic (water loving) head and a hydrophobic (water fearing) tail. If you pour some oil into water, it will form separate layers. learn how soap molecules with hydrophilic heads and. How Does Soap Work Reddit.
From www.tffn.net
The Chemistry of Soap How Does it Work? The Enlightened Mindset How Does Soap Work Reddit Soap is made of a chain, one end loves water, the other end doesn't like water, but this side surrounds oil and gunk. learn how soap molecules with hydrophilic heads and hydrophobic tails break apart the fatty acid. soap is a simple but powerful tool to fight germs and viruses. learn how soap cleans by acting as. How Does Soap Work Reddit.