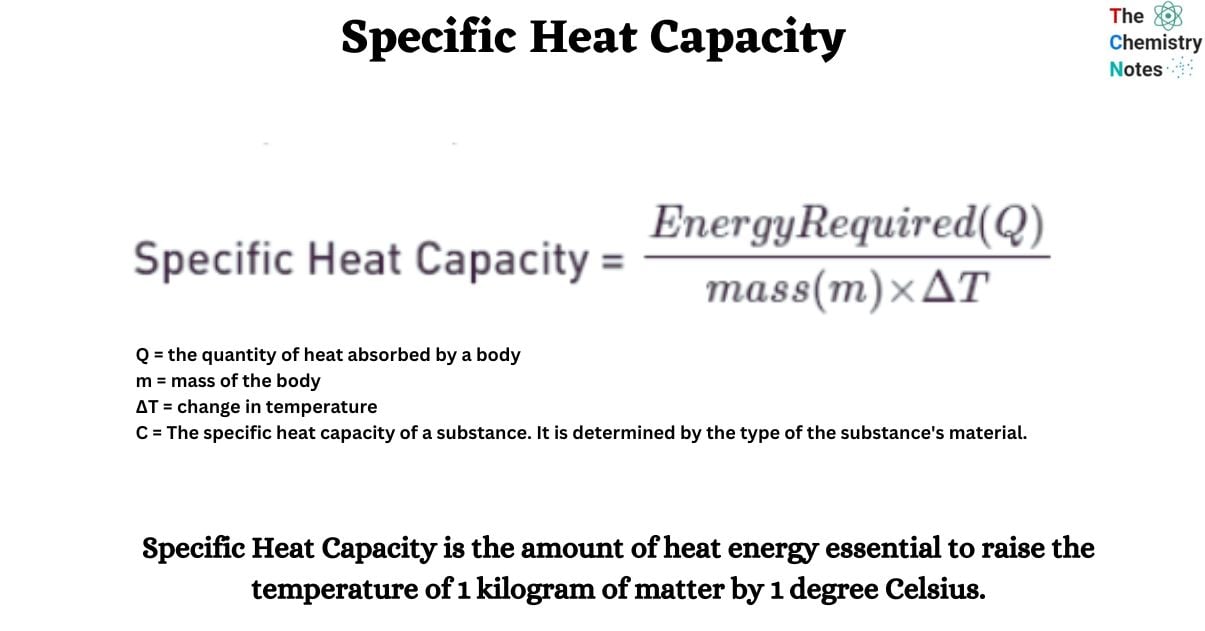
Equation Triangle For Specific Heat Capacity . Where q is the energy added and δt. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Use our specific heat calculator to calculate the energy required to achieve a desired temperature change, the mass, the change in temperature, or the specific heat capacity of a substance. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: Deduce which substance will have greatest. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. Define heat capacity and specific heat capacity and differentiate between the two terms. The units of specific heat capacity are j/(kg °c) or.
from scienceinfo.com
The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. Where q is the energy added and δt. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Deduce which substance will have greatest. Use our specific heat calculator to calculate the energy required to achieve a desired temperature change, the mass, the change in temperature, or the specific heat capacity of a substance. Define heat capacity and specific heat capacity and differentiate between the two terms. The units of specific heat capacity are j/(kg °c) or. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt).
Specific Heat Capacity Definition, Unit, Formula
Equation Triangle For Specific Heat Capacity Define heat capacity and specific heat capacity and differentiate between the two terms. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: Define heat capacity and specific heat capacity and differentiate between the two terms. The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Deduce which substance will have greatest. The units of specific heat capacity are j/(kg °c) or. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Use our specific heat calculator to calculate the energy required to achieve a desired temperature change, the mass, the change in temperature, or the specific heat capacity of a substance. Where q is the energy added and δt.
From ar.inspiredpencil.com
Triangle Chemistry Equation Triangle For Specific Heat Capacity Where q is the energy added and δt. The units of specific heat capacity are j/(kg °c) or. The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. It describes how much heat must be added to a unit of mass of a given substance. Equation Triangle For Specific Heat Capacity.
From jahamattbrown.blogspot.com
Specific Heat Capacity Definition Matt Brown Equation Triangle For Specific Heat Capacity The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. The units of specific heat capacity are j/(kg °c) or. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius.. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
Specific Heat Capacity Mr E Physics P3 AQA GCSE Physics YouTube Equation Triangle For Specific Heat Capacity Where q is the energy added and δt. The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. Use our specific heat calculator to calculate the energy required to achieve a desired temperature change, the mass, the change in temperature, or the specific heat capacity. Equation Triangle For Specific Heat Capacity.
From www.slideserve.com
PPT Learning Rearranging equation for Specific Heat Equation Triangle For Specific Heat Capacity It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The units of specific heat capacity are j/(kg °c) or. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Deduce which substance will have. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
Specific Heat 101 YouTube Equation Triangle For Specific Heat Capacity The units of specific heat capacity are j/(kg °c) or. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the.. Equation Triangle For Specific Heat Capacity.
From www.slideshare.net
Heat Capacity Equation Triangle For Specific Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Use our specific heat calculator to calculate the energy required to achieve a desired temperature change, the mass, the change in temperature, or the specific heat capacity of a substance. Deduce which substance will have greatest. The amount of energy. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity Equation Triangle For Specific Heat Capacity Deduce which substance will have greatest. Define heat capacity and specific heat capacity and differentiate between the two terms. The units of specific heat capacity are j/(kg °c) or. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Where q is the energy added and δt. Use our specific heat calculator. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
Specific Heat Capacity Short Example Solving for Mass YouTube Equation Triangle For Specific Heat Capacity The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: Where q is the energy added and δt. Define heat capacity and specific heat capacity and differentiate between the two terms. The definition of specific heat capacity of any substance is “the quantity of heat required to. Equation Triangle For Specific Heat Capacity.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula Equation Triangle For Specific Heat Capacity Define heat capacity and specific heat capacity and differentiate between the two terms. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
Specific heat capacity YouTube Equation Triangle For Specific Heat Capacity Where q is the energy added and δt. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The units of specific heat capacity are j/(kg °c) or. Define heat capacity and specific heat capacity and differentiate between the two terms. Deduce which substance will have greatest. It describes how much heat. Equation Triangle For Specific Heat Capacity.
From studylib.net
Heat Equation Equation Triangle For Specific Heat Capacity Where q is the energy added and δt. Use our specific heat calculator to calculate the energy required to achieve a desired temperature change, the mass, the change in temperature, or the specific heat capacity of a substance. The units of specific heat capacity are j/(kg °c) or. The definition of specific heat capacity of any substance is “the quantity. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial Equation Triangle For Specific Heat Capacity Define heat capacity and specific heat capacity and differentiate between the two terms. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Deduce which. Equation Triangle For Specific Heat Capacity.
From memim.com
Heat capacity ratio Equation Triangle For Specific Heat Capacity Where q is the energy added and δt. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: The definition of specific heat capacity of any substance is “the. Equation Triangle For Specific Heat Capacity.
From wordwall.net
Specific Latent Heat Equation Triangle Labelled diagram Equation Triangle For Specific Heat Capacity Where q is the energy added and δt. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Define heat capacity and specific heat capacity and differentiate between the two terms. The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
Thermodynamics Specific Heat Capacity Calculations YouTube Equation Triangle For Specific Heat Capacity Deduce which substance will have greatest. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Where q is the energy added and δt. Define heat capacity and specific. Equation Triangle For Specific Heat Capacity.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Equation Triangle For Specific Heat Capacity The units of specific heat capacity are j/(kg °c) or. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Define heat capacity and specific heat capacity. Equation Triangle For Specific Heat Capacity.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources Equation Triangle For Specific Heat Capacity Define heat capacity and specific heat capacity and differentiate between the two terms. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The units of specific heat capacity are j/(kg °c) or. It describes how much heat must be added to a unit of mass of a given substance. Equation Triangle For Specific Heat Capacity.
From www.slideserve.com
PPT Heat and Temperature PowerPoint Presentation, free download ID Equation Triangle For Specific Heat Capacity Deduce which substance will have greatest. Where q is the energy added and δt. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using. Equation Triangle For Specific Heat Capacity.
From www.grc.nasa.gov
Specific Heats Equation Triangle For Specific Heat Capacity The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. Where q is the energy added and δt. The units of specific heat capacity are j/(kg °c) or. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
Working Out SHC & SLH YouTube Equation Triangle For Specific Heat Capacity It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: The definition of specific heat capacity of any substance is “the quantity. Equation Triangle For Specific Heat Capacity.
From www.toppr.com
Specific Heat Formula Definition, Equations, Examples Equation Triangle For Specific Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Where q is the energy added and δt. The amount of energy needed to raise the temperature of a given mass by. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
Specific Heat Equation Stated Clearly YouTube Equation Triangle For Specific Heat Capacity Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Deduce which substance will have greatest. The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. The formula for specific heat capacity, c, of a substance with. Equation Triangle For Specific Heat Capacity.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems Equation Triangle For Specific Heat Capacity Deduce which substance will have greatest. Define heat capacity and specific heat capacity and differentiate between the two terms. The units of specific heat capacity are j/(kg °c) or. Where q is the energy added and δt. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation:. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Equation Triangle For Specific Heat Capacity The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: Deduce which substance will have greatest. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). It describes how much heat must be added to a unit. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
Q = mcΔT and Specific Heat IB Physics YouTube Equation Triangle For Specific Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the. Equation Triangle For Specific Heat Capacity.
From www.markedbyteachers.com
Specific Heat Capacity International Baccalaureate Physics Marked Equation Triangle For Specific Heat Capacity Where q is the energy added and δt. The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Deduce which. Equation Triangle For Specific Heat Capacity.
From quizzlibhofmann.z19.web.core.windows.net
Equation For Specific Heat Equation Triangle For Specific Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Define heat capacity and specific heat capacity and differentiate between the two terms. Deduce which substance will have greatest. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using. Equation Triangle For Specific Heat Capacity.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula Equation Triangle For Specific Heat Capacity The units of specific heat capacity are j/(kg °c) or. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: Use our. Equation Triangle For Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 Equation Triangle For Specific Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Use our specific heat calculator to calculate the energy required to achieve a desired temperature change, the mass, the change in temperature, or the specific heat capacity of a substance. The amount of energy needed to raise the temperature of. Equation Triangle For Specific Heat Capacity.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources Equation Triangle For Specific Heat Capacity Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The units of specific heat capacity are j/(kg °c) or. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the energy added and δt. Define heat capacity and. Equation Triangle For Specific Heat Capacity.
From www.youtube.com
GCSE Physics Revision "Specific Latent Heat" YouTube Equation Triangle For Specific Heat Capacity Use our specific heat calculator to calculate the energy required to achieve a desired temperature change, the mass, the change in temperature, or the specific heat capacity of a substance. Deduce which substance will have greatest. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The formula for specific heat capacity,. Equation Triangle For Specific Heat Capacity.
From www.mdpi.com
ChemEngineering Free FullText A Hill Equation for Solid Specific Equation Triangle For Specific Heat Capacity The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation: It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Energy transferred to increase the temperature of a substance by heating is. Equation Triangle For Specific Heat Capacity.
From slidetodoc.com
SPECIFIC HEAT Calculations SPECIFIC HEAT CAPACITY The amount Equation Triangle For Specific Heat Capacity The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The formula for specific heat capacity, c, of a substance with mass m, is c = q. Equation Triangle For Specific Heat Capacity.
From slidetodoc.com
Enthalpy Calculations And the specific heat equation Enthalpy Equation Triangle For Specific Heat Capacity It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The definition of specific heat capacity of any substance is “the quantity of heat required to change the temperature of a unit mass of the. The units of specific heat capacity are j/(kg °c) or.. Equation Triangle For Specific Heat Capacity.
From www.savemyexams.com
Specific Latent Heat AQA GCSE Physics Revision Notes 2018 Equation Triangle For Specific Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The definition of specific heat capacity of any substance is “the quantity of heat required. Equation Triangle For Specific Heat Capacity.