Lab Dilution Question . (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. Calculate the number of moles and the mass of the solute in each of the following solutions: How much of it do you need to prepare 50 ml of a. We are often concerned with. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of 0.750 m nacl on a shelf. Understand how stock solutions are used in the laboratory. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. I add 560 ml more water to it? Dilution m 1v 1=m 2v 2 1. Explain how concentrations can be changed in the lab. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of.
from www.studocu.com
1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Understand how stock solutions are used in the laboratory. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. We are often concerned with. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. I add 560 ml more water to it? How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. Calculate the number of moles and the mass of the solute in each of the following solutions:
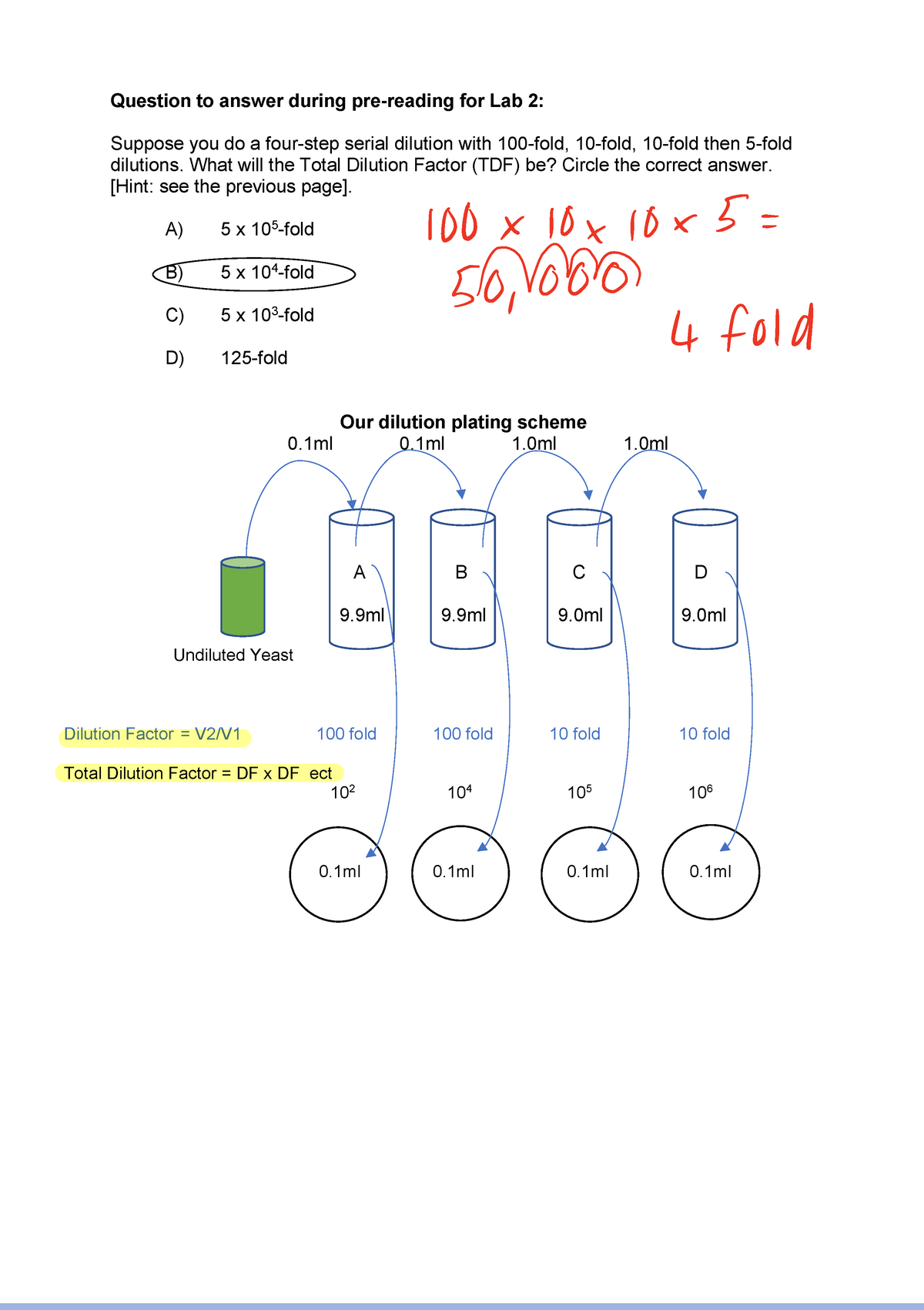
1. Serial Dilution Calculations Dilution Plating Questions Question
Lab Dilution Question Dilution m 1v 1=m 2v 2 1. Calculate the number of moles and the mass of the solute in each of the following solutions: Understand how stock solutions are used in the laboratory. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. Explain how concentrations can be changed in the lab. How much of it do you need to prepare 50 ml of a. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. I add 560 ml more water to it? A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. We are often concerned with. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if.
From quizgecko.com
Biology Lab Serial Dilutions & Plate Count Lab Dilution Question How much of it do you need to prepare 50 ml of a. We are often concerned with. I add 560 ml more water to it? Explain how concentrations can be changed in the lab. Calculate the number of moles and the mass of the solute in each of the following solutions: A dilution is a process where the concentration. Lab Dilution Question.
From www.solutionspile.com
[Solved] The Etest combines both the KirbyBauer disk diffu Lab Dilution Question Calculate the number of moles and the mass of the solute in each of the following solutions: We are often concerned with. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. Dilution m 1v 1=m 2v 2 1. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. 1) if i. Lab Dilution Question.
From www.chegg.com
Solved Determination of Copper in Ore Informal Lab reportppm Lab Dilution Question Dilution m 1v 1=m 2v 2 1. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. Understand how stock solutions are used in the laboratory. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. There’s a bottle of 0.750 m nacl on a shelf. We are often concerned with. How. Lab Dilution Question.
From www.youtube.com
Dilution Calculation Practice YouTube Lab Dilution Question I add 560 ml more water to it? Understand how stock solutions are used in the laboratory. Explain how concentrations can be changed in the lab. There’s a bottle of 0.750 m nacl on a shelf. Calculate the number of moles and the mass of the solute in each of the following solutions: 2) if i dilute 250 ml of. Lab Dilution Question.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Lab Dilution Question How much of it do you need to prepare 50 ml of a. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. We are often concerned with. I add 560 ml more water to it? (a) 2.00 l of 18.5 m h 2 so 4, concentrated. Lab Dilution Question.
From fyoezjbli.blob.core.windows.net
Dilution Technique For Blood Volume at Anne Cox blog Lab Dilution Question 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. How much of it do you need to prepare. Lab Dilution Question.
From quizgecko.com
Biology Lab Serial Dilutions & Plate Count Lab Dilution Question Dilution m 1v 1=m 2v 2 1. I add 560 ml more water to it? 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. There’s a bottle of 0.750 m nacl on a shelf. Explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. (a). Lab Dilution Question.
From hxegqvmtm.blob.core.windows.net
Dilution Method Definition at Ernest Alcazar blog Lab Dilution Question How much of it do you need to prepare 50 ml of a. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution m 1v 1=m 2v 2 1. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. We are often concerned with. 2) if i dilute 250. Lab Dilution Question.
From edubirdie.com
Lab 6 Determination of the Dye Concentration in A Edubirdie Lab Dilution Question Understand how stock solutions are used in the laboratory. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. How much of it do you need to prepare 50 ml of a. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. There’s a bottle of 0.750 m nacl on a shelf.. Lab Dilution Question.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Lab Dilution Question We are often concerned with. Calculate the number of moles and the mass of the solute in each of the following solutions: I add 560 ml more water to it? A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Understand how stock solutions are used in. Lab Dilution Question.
From treeprecision.weebly.com
3 Fold Serial Dilution treeprecision Lab Dilution Question There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2 1. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Calculate the number of moles and the mass of the solute in each of the following solutions: How much of. Lab Dilution Question.
From pressurewashingresource.com
Chem dilution chart 2 by Chemical/Chemistry Lab Dilution Question Calculate the number of moles and the mass of the solute in each of the following solutions: 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. I add 560 ml more water to it? There’s a bottle of 0.750 m nacl on a shelf. Understand how stock solutions are used in. Lab Dilution Question.
From quizgecko.com
Biology Lab Serial Dilutions & Plate Count Lab Dilution Question Dilution m 1v 1=m 2v 2 1. Calculate the number of moles and the mass of the solute in each of the following solutions: There’s a bottle of 0.750 m nacl on a shelf. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. We are often concerned with. How much of it do you need to prepare. Lab Dilution Question.
From www.pinterest.es
Serial dilutions Lab humor, Laboratory humor Lab Dilution Question (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. I add 560 ml more water to it? We are often concerned with. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Understand. Lab Dilution Question.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Lab Dilution Question Explain how concentrations can be changed in the lab. Calculate the number of moles and the mass of the solute in each of the following solutions: Dilution m 1v 1=m 2v 2 1. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. 1) if i have 340 ml of a 0.5 m nabr. Lab Dilution Question.
From ai.hubermanlab.com
Navigating SAFE Agreements Ask Huberman Lab Lab Dilution Question Dilution m 1v 1=m 2v 2 1. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of. Lab Dilution Question.
From exooxjrsa.blob.core.windows.net
Dilution Calculator Antibody at Jennifer Casale blog Lab Dilution Question Explain how concentrations can be changed in the lab. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. We are often concerned with. How much of it do you need to prepare 50 ml of a. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume. Lab Dilution Question.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Lab Dilution Question (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. Explain how concentrations can be changed in the lab. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. Calculate the number of moles and. Lab Dilution Question.
From www.chegg.com
Solved Serial dilution is a common technique used in Lab Dilution Question Calculate the number of moles and the mass of the solute in each of the following solutions: Dilution m 1v 1=m 2v 2 1. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. A dilution is a process where the concentration of a solution is lowered by adding solvent to the. Lab Dilution Question.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Lab Dilution Question How much of it do you need to prepare 50 ml of a. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. Explain how concentrations can be changed in the lab. I add 560. Lab Dilution Question.
From www.etsy.com
Dilution Guide Serial & Normal Clinical Laboratory Dilutions Mlt/mls/mt Lab Dilution Question (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. Explain how concentrations can be changed in the lab.. Lab Dilution Question.
From www.chegg.com
PLEASE ANSWER FULL DETAIL LOOK AT IMAGE!! 1. The Lab Dilution Question Dilution m 1v 1=m 2v 2 1. I add 560 ml more water to it? (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. There’s a bottle of 0.750 m nacl on a shelf. Calculate the number of moles and the mass of the solute in each of the following solutions: We are often concerned with. 2). Lab Dilution Question.
From edubirdie.com
Lab 6 Determination of the Dye Concentration in A Edubirdie Lab Dilution Question A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. We are often concerned with. Understand how stock solutions are used in the laboratory. Calculate the number of moles and the mass of the solute in each of the following solutions: How much of it do you. Lab Dilution Question.
From edubirdie.com
Lab 6 Determination of the Dye Concentration in A Edubirdie Lab Dilution Question Understand how stock solutions are used in the laboratory. Dilution m 1v 1=m 2v 2 1. How much of it do you need to prepare 50 ml of a. I add 560 ml more water to it? 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. We are often concerned with. Calculate the. Lab Dilution Question.
From edubirdie.com
Lab 6 Determination of the Dye Concentration in A Edubirdie Lab Dilution Question Understand how stock solutions are used in the laboratory. Calculate the number of moles and the mass of the solute in each of the following solutions: There’s a bottle of 0.750 m nacl on a shelf. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. (a) 2.00 l of 18.5 m. Lab Dilution Question.
From classnotes.guru
Salmonella Bacteriology Class Notes Lab Dilution Question There’s a bottle of 0.750 m nacl on a shelf. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. Calculate the number of moles and the mass of the solute in each of the following solutions: Understand how stock solutions are used in the laboratory. How much of it do you need to. Lab Dilution Question.
From www.answersarena.com
[Solved] The process by which a semipermeable membrane allo Lab Dilution Question I add 560 ml more water to it? Understand how stock solutions are used in the laboratory. How much of it do you need to prepare 50 ml of a. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. Dilution m 1v 1=m 2v 2 1. 2) if i dilute 250 ml of 0.10 m lithium acetate. Lab Dilution Question.
From hxefeasye.blob.core.windows.net
Blood Dilution Procedure at Brett Rawls blog Lab Dilution Question I add 560 ml more water to it? (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. 1). Lab Dilution Question.
From www.chegg.com
Solved Prepare a 2 fold serial dilution of your standard up Lab Dilution Question I add 560 ml more water to it? How much of it do you need to prepare 50 ml of a. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution m 1v 1=m 2v 2 1. We are often concerned with. Calculate the number of. Lab Dilution Question.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Lab Dilution Question Understand how stock solutions are used in the laboratory. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. Calculate the number of moles and the mass of the solute in each of the following solutions: How much of it do you need to prepare 50 ml of a. We are often concerned with.. Lab Dilution Question.
From edubirdie.com
Lab 6 Determination of the Dye Concentration in A Edubirdie Lab Dilution Question 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of 0.750 m nacl on a shelf. Calculate the number of moles and the mass of the solute in each of the following solutions: We are often concerned with. 2) if i dilute 250 ml of 0.10 m lithium. Lab Dilution Question.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Lab Dilution Question I add 560 ml more water to it? A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. Calculate the number of moles and the mass of the solute in each. Lab Dilution Question.
From labrobot.com
Dilutions décimales Microbiologie alimentaire Système Dilucup Lab Dilution Question Dilution m 1v 1=m 2v 2 1. Calculate the number of moles and the mass of the solute in each of the following solutions: Understand how stock solutions are used in the laboratory. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. (a) 2.00 l of 18.5 m h 2 so 4, concentrated. Lab Dilution Question.