Bromomethane Intermolecular Forces . There must be another factor that compensates for the difference in numbers. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. The forces that are exercised between molecules are called intermolecular forces; The following is the order from lowest boiling point to highest based on the types of forces these compounds have: It is a colorless gas that consists of one. Those inside of a molecule are intramolecular forces. Those inside of a molecule are intramolecular forces. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with. The forces that are exercised between molecules are called intermolecular forces;
from www.doubtnut.com
Those inside of a molecule are intramolecular forces. The forces that are exercised between molecules are called intermolecular forces; The following is the order from lowest boiling point to highest based on the types of forces these compounds have: There must be another factor that compensates for the difference in numbers. The forces that are exercised between molecules are called intermolecular forces; It is a colorless gas that consists of one. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. Those inside of a molecule are intramolecular forces. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with.
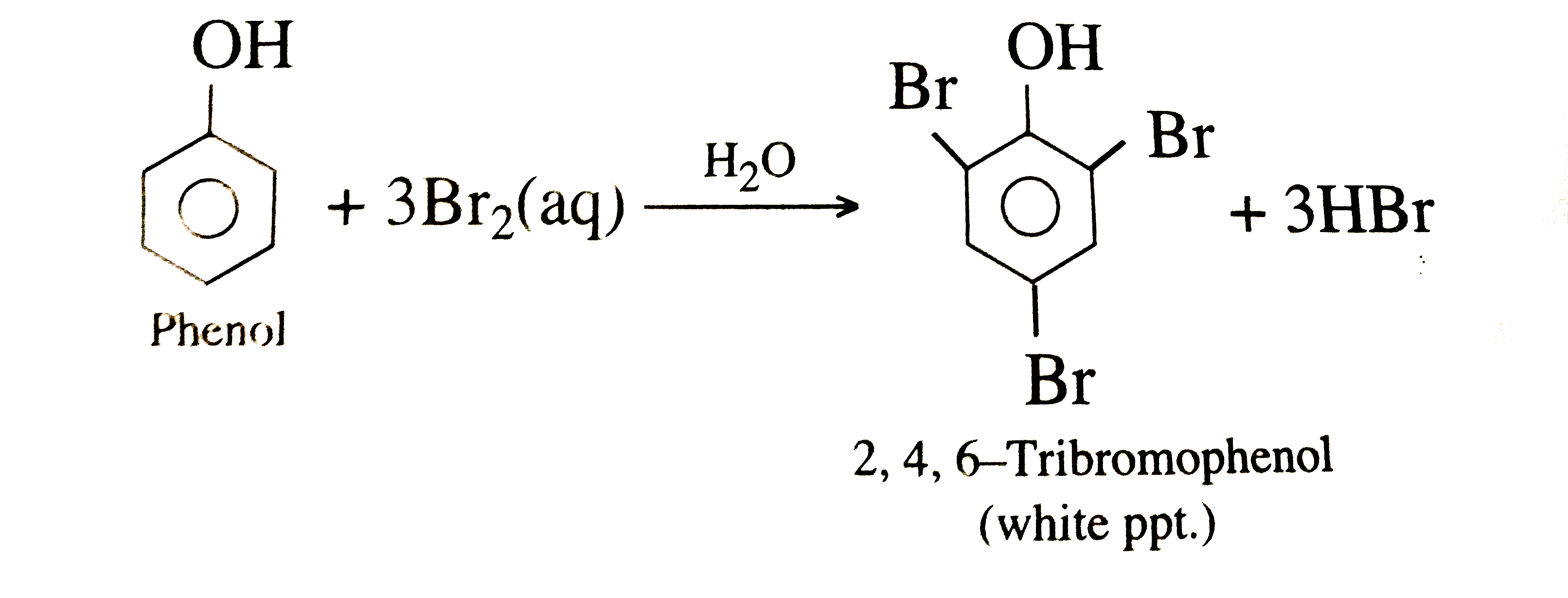
Discuss the mechanism of alkaline hydrolysisi of bromomethane. How i
Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. There must be another factor that compensates for the difference in numbers. The forces that are exercised between molecules are called intermolecular forces; The following is the order from lowest boiling point to highest based on the types of forces these compounds have: It is a colorless gas that consists of one. The forces that are exercised between molecules are called intermolecular forces; Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with. Those inside of a molecule are intramolecular forces. Those inside of a molecule are intramolecular forces.
From slideplayer.com
Intermolecular Forces Notes ppt download Bromomethane Intermolecular Forces There must be another factor that compensates for the difference in numbers. Those inside of a molecule are intramolecular forces. The following is the order from lowest boiling point to highest based on the types of forces these compounds have: Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the. Bromomethane Intermolecular Forces.
From sciencenotes.org
Intermolecular Forces in Chemistry Bromomethane Intermolecular Forces Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with. The following is the order from lowest boiling point to highest based on the types of forces these compounds have: The forces that are exercised between molecules are. Bromomethane Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. Those inside of a molecule are intramolecular forces. It is a colorless gas that consists of one. There must be another factor that compensates for the difference in numbers. The following is the order from lowest boiling. Bromomethane Intermolecular Forces.
From materialfulldelation.z13.web.core.windows.net
Intermolecular Forces Explained Simply Bromomethane Intermolecular Forces The following is the order from lowest boiling point to highest based on the types of forces these compounds have: It is a colorless gas that consists of one. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness. Bromomethane Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download Bromomethane Intermolecular Forces The forces that are exercised between molecules are called intermolecular forces; Those inside of a molecule are intramolecular forces. It is a colorless gas that consists of one. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with.. Bromomethane Intermolecular Forces.
From mungfali.com
Three Types Of Intermolecular Forces Bromomethane Intermolecular Forces There must be another factor that compensates for the difference in numbers. It is a colorless gas that consists of one. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. Those inside of a molecule are intramolecular forces. The forces that are exercised between molecules are called intermolecular forces; The following is the order. Bromomethane Intermolecular Forces.
From www.slideshare.net
Intermolecular forces Bromomethane Intermolecular Forces It is a colorless gas that consists of one. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. Those inside of a molecule are intramolecular forces. There must be another factor that compensates for the difference in numbers. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of. Bromomethane Intermolecular Forces.
From evulpo.com
Intermolecular forces types and examples Chemistry Explanation Bromomethane Intermolecular Forces Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. Those inside of a molecule are intramolecular forces. There must be another factor that compensates for the difference in numbers. It is a colorless gas that consists of one. The following is the order from lowest boiling point to highest based on the types of. Bromomethane Intermolecular Forces.
From www.dreamstime.com
3D Image of Bromomethane Skeletal Formula Stock Illustration Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. The forces that are exercised between molecules are called intermolecular forces; It is a colorless gas that consists of one. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with.. Bromomethane Intermolecular Forces.
From www.dreamstime.com
Bromomethane Molecular Structure Isolated on White Stock Illustration Bromomethane Intermolecular Forces It is a colorless gas that consists of one. The following is the order from lowest boiling point to highest based on the types of forces these compounds have: Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. Those inside of a molecule are intramolecular forces. The forces that are exercised between molecules are. Bromomethane Intermolecular Forces.
From www.dreamstime.com
Bromomethane Molecule. Isolated Molecular Model. 3D Rendering Stock Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with. There must be another factor that compensates for the difference in numbers. The forces that are exercised between molecules are. Bromomethane Intermolecular Forces.
From www.dreamstime.com
Molecular Model of Bromomethane, 3D Rendering Stock Illustration Bromomethane Intermolecular Forces The forces that are exercised between molecules are called intermolecular forces; It is a colorless gas that consists of one. The following is the order from lowest boiling point to highest based on the types of forces these compounds have: Those inside of a molecule are intramolecular forces. Those inside of a molecule are intramolecular forces. Bromomethane bp = 4°. Bromomethane Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. The forces that are exercised between molecules are called intermolecular forces; Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with. The forces that are exercised between molecules are called. Bromomethane Intermolecular Forces.
From www.dreamstime.com
Bromomethane Methyl Bromide Stock Vector Illustration of atoms Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. There must be another factor that compensates for the difference in numbers. It is a colorless gas that consists of one. Those inside of a molecule are intramolecular forces. Bromomethane bp = 4° boiling points of alkyl. Bromomethane Intermolecular Forces.
From www.dreamstime.com
Bromomethane Molecular Structure, 3d Model Molecule, Methyl Bromide Bromomethane Intermolecular Forces Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. The forces that are exercised between molecules are called intermolecular forces; Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with. There must. Bromomethane Intermolecular Forces.
From www.dreamstime.com
Chemistry Illustration of Bromomethane Blue Stock Illustration Bromomethane Intermolecular Forces Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with. The following is the order from lowest boiling point to highest based on the types of forces these compounds have: The forces that are exercised between molecules are. Bromomethane Intermolecular Forces.
From www.freepng.fr
Du Bromométhane, Bromure De, Chimie PNG Du Bromométhane, Bromure De Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. Those inside of a molecule are intramolecular forces. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. The forces that are exercised between molecules are called intermolecular forces; It is a colorless gas that consists of one. Bromomethane bp = 4° boiling points of alkyl fluorides. Bromomethane Intermolecular Forces.
From www.dreamstime.com
Bromomethane Molecule, Molecular Structure, Methyl Bromide, Ball and Bromomethane Intermolecular Forces There must be another factor that compensates for the difference in numbers. Those inside of a molecule are intramolecular forces. The forces that are exercised between molecules are called intermolecular forces; The forces that are exercised between molecules are called intermolecular forces; The following is the order from lowest boiling point to highest based on the types of forces these. Bromomethane Intermolecular Forces.
From www.youtube.com
CH3Br Lewis Structure (Bromomethane) YouTube Bromomethane Intermolecular Forces The forces that are exercised between molecules are called intermolecular forces; Those inside of a molecule are intramolecular forces. Those inside of a molecule are intramolecular forces. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. There must be another factor that compensates for the difference in numbers. The following is the order from. Bromomethane Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download Bromomethane Intermolecular Forces It is a colorless gas that consists of one. The forces that are exercised between molecules are called intermolecular forces; Those inside of a molecule are intramolecular forces. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine, the tightness with.. Bromomethane Intermolecular Forces.
From scienceinfo.com
Intermolecular Forces Definition and Types Bromomethane Intermolecular Forces The forces that are exercised between molecules are called intermolecular forces; There must be another factor that compensates for the difference in numbers. The forces that are exercised between molecules are called intermolecular forces; The following is the order from lowest boiling point to highest based on the types of forces these compounds have: Those inside of a molecule are. Bromomethane Intermolecular Forces.
From www.istockphoto.com
Structure Moléculaire De Bromométhane Isolée Sur Blanc Vecteurs libres Bromomethane Intermolecular Forces The forces that are exercised between molecules are called intermolecular forces; It is a colorless gas that consists of one. Those inside of a molecule are intramolecular forces. The following is the order from lowest boiling point to highest based on the types of forces these compounds have: The forces that are exercised between molecules are called intermolecular forces; There. Bromomethane Intermolecular Forces.
From stock.adobe.com
Bromomethane molecule. Ballandstick molecular model. Chemistry Bromomethane Intermolecular Forces The forces that are exercised between molecules are called intermolecular forces; The following is the order from lowest boiling point to highest based on the types of forces these compounds have: It is a colorless gas that consists of one. Those inside of a molecule are intramolecular forces. Bromomethane, also known as methyl bromide, is a chemical compound with the. Bromomethane Intermolecular Forces.
From www.alamy.com
Bromomethane hires stock photography and images Alamy Bromomethane Intermolecular Forces There must be another factor that compensates for the difference in numbers. The following is the order from lowest boiling point to highest based on the types of forces these compounds have: Those inside of a molecule are intramolecular forces. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the. Bromomethane Intermolecular Forces.
From www.doubtnut.com
Discuss the mechanism of alkaline hydrolysisi of bromomethane. How i Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. It is a colorless gas that consists of one. Those inside of a molecule are intramolecular forces. The following is the order from lowest boiling point to highest based on the types of forces these compounds have: There must be another factor that compensates for the difference in numbers. The forces that. Bromomethane Intermolecular Forces.
From www.shutterstock.com
Bromomethane Molecule 3d Rendering Flat Molecular Stock Illustration Bromomethane Intermolecular Forces The following is the order from lowest boiling point to highest based on the types of forces these compounds have: Those inside of a molecule are intramolecular forces. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. There must be another factor that compensates for the difference in numbers. Bromomethane bp = 4° boiling. Bromomethane Intermolecular Forces.
From www.dreamstime.com
Bromomethane Molecular Structure Isolated on Black Stock Illustration Bromomethane Intermolecular Forces The forces that are exercised between molecules are called intermolecular forces; It is a colorless gas that consists of one. The forces that are exercised between molecules are called intermolecular forces; Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine,. Bromomethane Intermolecular Forces.
From www.futura-sciences.com
Définition Bromure de méthyle Bromométhane Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. Those inside of a molecule are intramolecular forces. The following is the order from lowest boiling point to highest based on the types of forces these compounds have: Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. The forces that are exercised between molecules are called. Bromomethane Intermolecular Forces.
From webmis.highland.cc.il.us
Intermolecular Forces Bromomethane Intermolecular Forces There must be another factor that compensates for the difference in numbers. The forces that are exercised between molecules are called intermolecular forces; Those inside of a molecule are intramolecular forces. Those inside of a molecule are intramolecular forces. The forces that are exercised between molecules are called intermolecular forces; It is a colorless gas that consists of one. The. Bromomethane Intermolecular Forces.
From www.dreamstime.com
Bromomethane Molecule, Structural Chemical Formula, Ballandstick Bromomethane Intermolecular Forces The forces that are exercised between molecules are called intermolecular forces; The forces that are exercised between molecules are called intermolecular forces; Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. Those inside of a molecule are intramolecular forces. The following is the order from lowest boiling point to highest based on the types. Bromomethane Intermolecular Forces.
From www.istockphoto.com
Structure Moléculaire De Bromométhane Disolement Sur Le Blanc Vecteurs Bromomethane Intermolecular Forces There must be another factor that compensates for the difference in numbers. The forces that are exercised between molecules are called intermolecular forces; Those inside of a molecule are intramolecular forces. The forces that are exercised between molecules are called intermolecular forces; Those inside of a molecule are intramolecular forces. Bromomethane, also known as methyl bromide, is a chemical compound. Bromomethane Intermolecular Forces.
From jsmithmoore.com
Intermolecular forces Bromomethane Intermolecular Forces Those inside of a molecule are intramolecular forces. Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. The forces that are exercised between molecules are called intermolecular forces; The forces that are exercised between molecules are called intermolecular forces; Those inside of a molecule are intramolecular forces. It is a colorless gas that consists. Bromomethane Intermolecular Forces.
From slideplayer.com
Chapter 11 Intermolecular Forces, Liquids and Solids ppt download Bromomethane Intermolecular Forces Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. Those inside of a molecule are intramolecular forces. It is a colorless gas that consists of one. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of. Bromomethane Intermolecular Forces.
From www.chegg.com
Solved Identify the highest occupied molecular orbital of Bromomethane Intermolecular Forces The forces that are exercised between molecules are called intermolecular forces; Those inside of a molecule are intramolecular forces. There must be another factor that compensates for the difference in numbers. Bromomethane bp = 4° boiling points of alkyl fluorides are lower than those of hydrocarbons of comparable molecular weight the difference is due to the small size of fluorine,. Bromomethane Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download Bromomethane Intermolecular Forces Bromomethane, also known as methyl bromide, is a chemical compound with the molecular formula ch₃br. There must be another factor that compensates for the difference in numbers. The forces that are exercised between molecules are called intermolecular forces; It is a colorless gas that consists of one. The following is the order from lowest boiling point to highest based on. Bromomethane Intermolecular Forces.