What Is A Shared Pair Of Electrons . Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. The electrons involved are in the outer shells of the atoms. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. However, in many molecules atoms attain. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. An atom that shares one or more of its electrons will complete its outer shell.
from www.nagwa.com
Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. An atom that shares one or more of its electrons will complete its outer shell. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. However, in many molecules atoms attain. The electrons involved are in the outer shells of the atoms. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle.
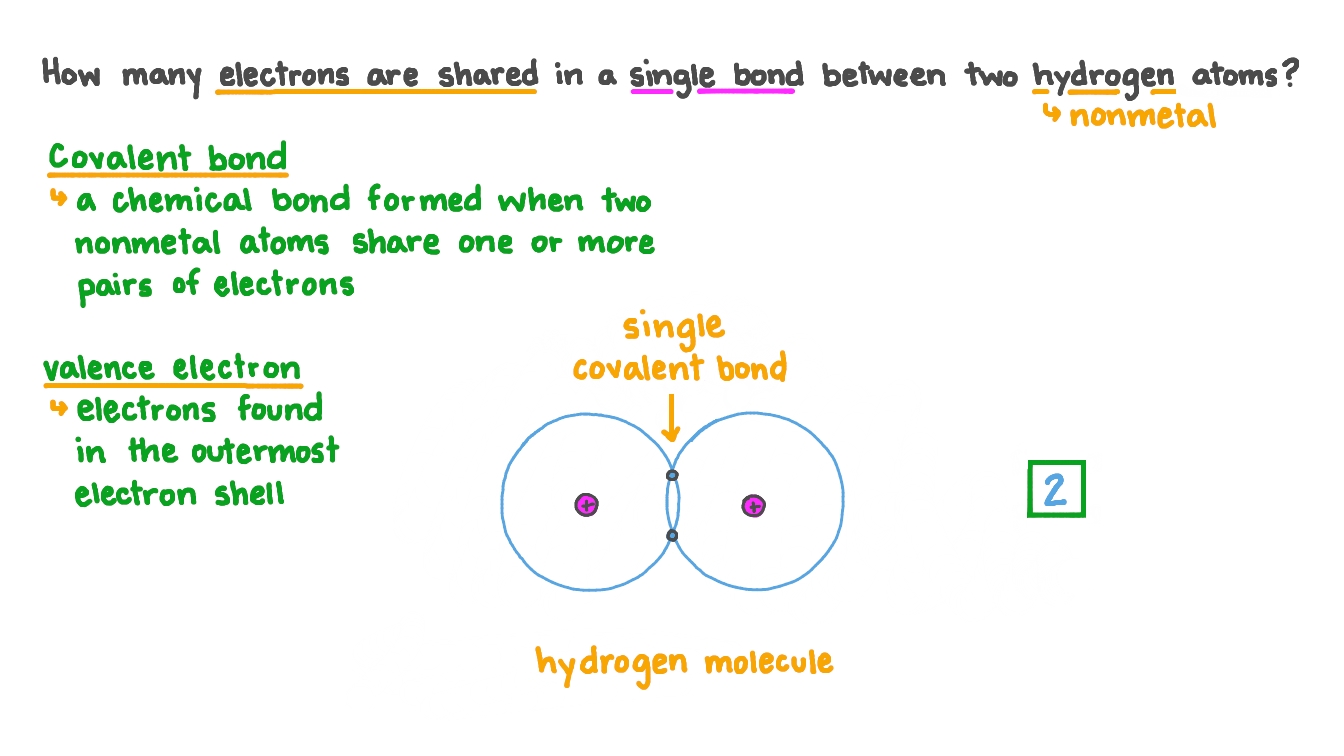
Question Video Determining How Many Electrons Are Shared in a Single
What Is A Shared Pair Of Electrons The electrons involved are in the outer shells of the atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. An atom that shares one or more of its electrons will complete its outer shell. However, in many molecules atoms attain. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. The electrons involved are in the outer shells of the atoms.
From byjus.com
10.The difference between number of shared electron pairs and unpaired What Is A Shared Pair Of Electrons The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. An atom that shares one or more of its electrons will complete its outer shell.. What Is A Shared Pair Of Electrons.
From www.numerade.com
SOLVED Question 2 Select all of the below statements that accurately What Is A Shared Pair Of Electrons When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. An atom that shares one or more of its electrons will complete its outer shell.. What Is A Shared Pair Of Electrons.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules What Is A Shared Pair Of Electrons When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. The electrons involved are in the outer shells of the atoms. However, in. What Is A Shared Pair Of Electrons.
From www.youtube.com
How To Determine The Number of Paired and Unpaired Electrons YouTube What Is A Shared Pair Of Electrons An atom that shares one or more of its electrons will complete its outer shell. However, in many molecules atoms attain. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. Electron sharing is the sharing of the outermost electrons between two or more atoms. What Is A Shared Pair Of Electrons.
From chemistry.stackexchange.com
organic chemistry Why is the lone pair of pyridine's nitrogen atom What Is A Shared Pair Of Electrons A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. An atom that shares one or more of its electrons will complete its outer shell. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. The electrons involved are in the. What Is A Shared Pair Of Electrons.
From gianni-has-frederick.blogspot.com
Lone Pair of Electrons GiannihasFrederick What Is A Shared Pair Of Electrons An atom that shares one or more of its electrons will complete its outer shell. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. The electrons involved are in the. What Is A Shared Pair Of Electrons.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille What Is A Shared Pair Of Electrons When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Electron sharing is the sharing of the outermost electrons between two or more atoms without the. What Is A Shared Pair Of Electrons.
From www.nagwa.com
Question Video Determining How Many Electrons Are Shared in a Single What Is A Shared Pair Of Electrons When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. The sharing of a pair of electrons represents a single covalent bond, usually. What Is A Shared Pair Of Electrons.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together What Is A Shared Pair Of Electrons When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electron sharing is the sharing of the outermost electrons between two or more atoms without. What Is A Shared Pair Of Electrons.
From depositphotos.com
What Happens Atoms Bond Infographic Diagram Showing How Electrons What Is A Shared Pair Of Electrons However, in many molecules atoms attain. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. When combining with another nitrogen atom to form a diatomic molecule, the three. What Is A Shared Pair Of Electrons.
From www.slideshare.net
Connect The Dots What Is A Shared Pair Of Electrons Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as. What Is A Shared Pair Of Electrons.
From www.slideserve.com
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download What Is A Shared Pair Of Electrons However, in many molecules atoms attain. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. When combining with another nitrogen atom to form a diatomic molecule, the. What Is A Shared Pair Of Electrons.
From www.slideserve.com
PPT Unit 7 Bonding & Molecular Geometry PowerPoint Presentation What Is A Shared Pair Of Electrons Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between. What Is A Shared Pair Of Electrons.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID What Is A Shared Pair Of Electrons Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs. What Is A Shared Pair Of Electrons.
From www.expii.com
Lone vs. Bonding Electron Pairs — Comparison & Importance Expii What Is A Shared Pair Of Electrons However, in many molecules atoms attain. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Electron sharing is the sharing of the outermost electrons between. What Is A Shared Pair Of Electrons.
From klaxanybf.blob.core.windows.net
What Is Sharing Of Electrons at Stanley Killinger blog What Is A Shared Pair Of Electrons Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. However,. What Is A Shared Pair Of Electrons.
From emedia.rmit.edu.au
Covalent bonds Learning Lab What Is A Shared Pair Of Electrons The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the. What Is A Shared Pair Of Electrons.
From www.slideserve.com
PPT 8.1 Covalent Bonds PowerPoint Presentation, free download ID What Is A Shared Pair Of Electrons However, in many molecules atoms attain. An atom that shares one or more of its electrons will complete its outer shell. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. Electron sharing is the sharing of the outermost electrons between two or more atoms. What Is A Shared Pair Of Electrons.
From www.blitznotes.org
Bonding What Is A Shared Pair Of Electrons The electrons involved are in the outer shells of the atoms. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. An atom that shares one or more of its electrons will complete its outer shell. The sharing of a pair of electrons represents a single covalent bond, usually just referred. What Is A Shared Pair Of Electrons.
From www.youtube.com
Lewis Dot Structures How To Calculate The Number of Lone Pairs Using What Is A Shared Pair Of Electrons The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. The electrons involved are in the outer shells of the atoms. An atom that shares one or more of its electrons will complete its outer shell. A covalent bond is a chemical bond that involves the sharing of electrons to. What Is A Shared Pair Of Electrons.
From klaxanybf.blob.core.windows.net
What Is Sharing Of Electrons at Stanley Killinger blog What Is A Shared Pair Of Electrons The electrons involved are in the outer shells of the atoms. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. When combining with another nitrogen atom to form a. What Is A Shared Pair Of Electrons.
From www.blog44.ca
Science Unit 3 Chemistry From the Mind of Isabelle What Is A Shared Pair Of Electrons However, in many molecules atoms attain. The electrons involved are in the outer shells of the atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. When. What Is A Shared Pair Of Electrons.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.44 Know that a Covalent Bond is Formed Between What Is A Shared Pair Of Electrons An atom that shares one or more of its electrons will complete its outer shell. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Paired. What Is A Shared Pair Of Electrons.
From brainly.in
Differentiate between the following with a suitable example1.)Lone What Is A Shared Pair Of Electrons Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. The electrons involved are in the outer shells of the atoms. Electron sharing is the sharing of the outermost electrons between two. What Is A Shared Pair Of Electrons.
From klaxanybf.blob.core.windows.net
What Is Sharing Of Electrons at Stanley Killinger blog What Is A Shared Pair Of Electrons However, in many molecules atoms attain. An atom that shares one or more of its electrons will complete its outer shell. The electrons involved are in the outer shells of the atoms. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. Paired electrons refer to two electrons. What Is A Shared Pair Of Electrons.
From www.slideserve.com
PPT 8.1 Covalent Bonds PowerPoint Presentation, free download ID What Is A Shared Pair Of Electrons Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. An. What Is A Shared Pair Of Electrons.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID1698471 What Is A Shared Pair Of Electrons A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form. What Is A Shared Pair Of Electrons.
From www.science-revision.co.uk
Covalent bonding What Is A Shared Pair Of Electrons When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. The electrons involved are in the outer shells of the atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. An atom that shares. What Is A Shared Pair Of Electrons.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts What Is A Shared Pair Of Electrons When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. However, in many molecules atoms attain. Paired electrons refer to two electrons occupying the same. What Is A Shared Pair Of Electrons.
From www.slideserve.com
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation ID5979513 What Is A Shared Pair Of Electrons Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. The electrons involved are in the outer shells of the atoms. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. An atom that shares one or. What Is A Shared Pair Of Electrons.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I What Is A Shared Pair Of Electrons Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. An atom that shares one or more of its electrons will complete its outer shell. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. A covalent. What Is A Shared Pair Of Electrons.
From socratic.org
Question 4e4a3 Socratic What Is A Shared Pair Of Electrons The electrons involved are in the outer shells of the atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. Electron sharing is the. What Is A Shared Pair Of Electrons.
From byjus.com
calculate total no.,shared pairs,unshared pairs and no. of valance What Is A Shared Pair Of Electrons A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. An atom that shares one or more of its electrons will complete its outer shell. Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. The electrons involved are in the outer shells. What Is A Shared Pair Of Electrons.
From askfilo.com
Triple covalent bond When 3 pairs of electrons are shared. forey (1).. What Is A Shared Pair Of Electrons Paired electrons refer to two electrons occupying the same orbital with opposite spins, following the pauli exclusion principle. However, in many molecules atoms attain. The electrons involved are in the outer shells of the atoms. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons to form. The sharing of. What Is A Shared Pair Of Electrons.
From users.highland.edu
Lewis Structures and Covalent Bonding What Is A Shared Pair Of Electrons When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of. An atom that shares one or more of its electrons will complete its outer shell. Electron sharing is the sharing of the outermost electrons between two or more atoms without the complete transfer of electrons. What Is A Shared Pair Of Electrons.