Respiratory Assist Device Guidelines . respiratory assist device (rad) qualifying guidelines. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months.
from healthandwillness.org
respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) qualifying guidelines.
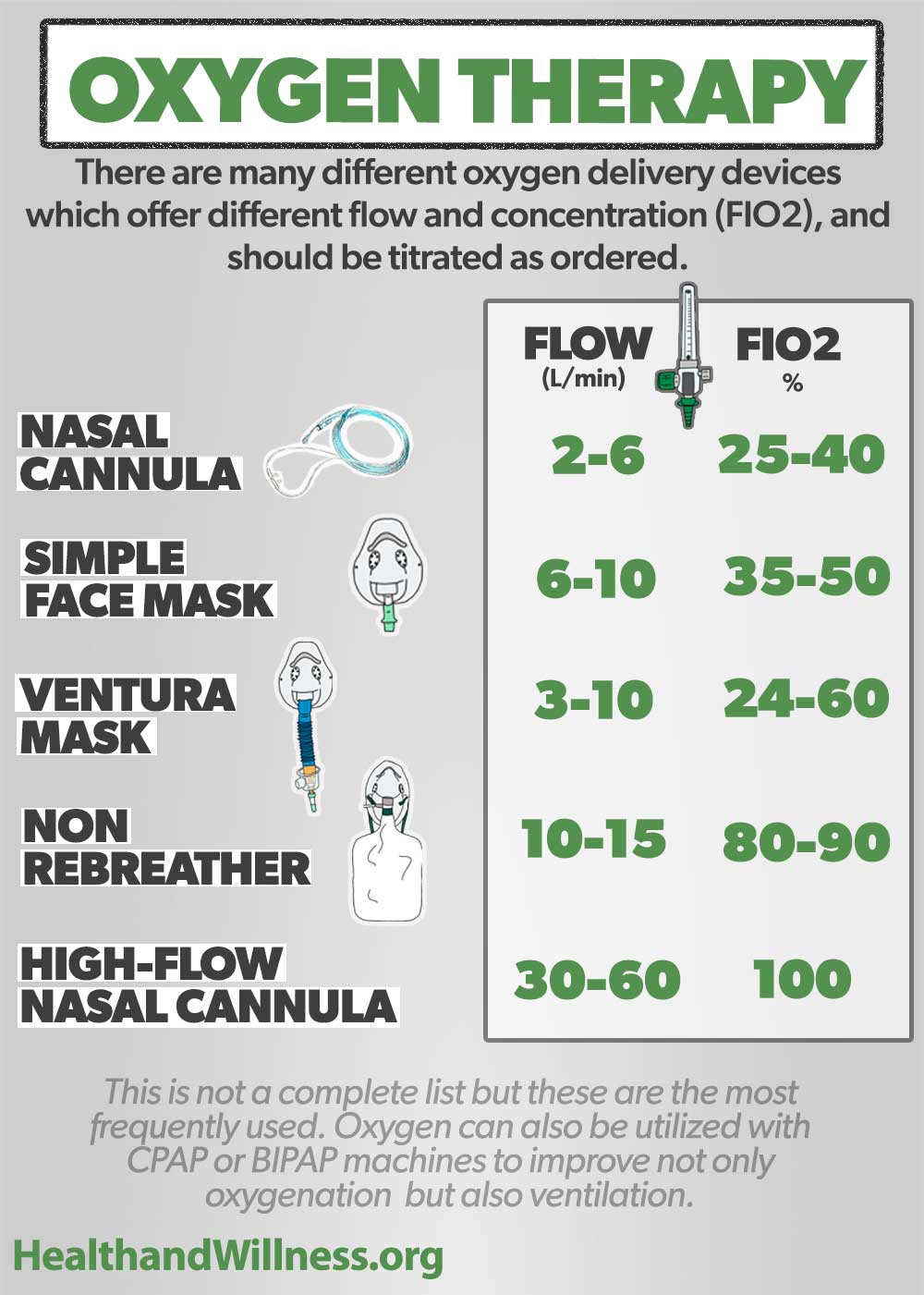
Oxygen Delivery Devices and Flow Rates Health And Willness
Respiratory Assist Device Guidelines respiratory assist device (rad) qualifying guidelines. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. respiratory assist device (rad) qualifying guidelines. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single.
From bmjopenrespres.bmj.com
British Thoracic Society/Intensive Care Society Guideline for the Respiratory Assist Device Guidelines respiratory assist device (rad) qualifying guidelines. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of. Respiratory Assist Device Guidelines.
From www.scribd.com
GuidelineBased Management of Acute Respiratory Failure and Acute Respiratory Assist Device Guidelines an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. a respiratory assist device (rad) without. Respiratory Assist Device Guidelines.
From cemcdyfx.blob.core.windows.net
Nasal HighFlow Oxygen Therapy Guidelines at James Little blog Respiratory Assist Device Guidelines an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) qualifying guidelines. respiratory assist devices include devices intended to help patients in need of support. Respiratory Assist Device Guidelines.
From news.engineering.pitt.edu
Hemolung® RAS Used to Treat More than 75 COVID19 Patients Respiratory Assist Device Guidelines a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist. Respiratory Assist Device Guidelines.
From www.bmj.com
Respiratory support The BMJ Respiratory Assist Device Guidelines a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. respiratory assist device (rad) qualifying guidelines. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction. Respiratory Assist Device Guidelines.
From www.youtube.com
Respiratory Assist Device (RAD) Initial 12Week Coverage Guidelines Respiratory Assist Device Guidelines respiratory assist device (rad) qualifying guidelines. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. the information in this letter is intended to assist you in documenting that your patient meets. Respiratory Assist Device Guidelines.
From docslib.org
Respiratory Inhaler Identification Chart Examples of Different Inhaler Respiratory Assist Device Guidelines respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. respiratory assist device (rad) qualifying guidelines. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. respiratory assist devices include. Respiratory Assist Device Guidelines.
From studylib.net
Respiratory Assist Device Respiratory Assist Device Guidelines an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. . Respiratory Assist Device Guidelines.
From www.researchgate.net
Hemolung ® Respiratory Assist System. Download Scientific Diagram Respiratory Assist Device Guidelines the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. respiratory assist device (rad) qualifying guidelines. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. respiratory. Respiratory Assist Device Guidelines.
From the-strategists-network.com
Hemolung Respiratory Assist System the Strategists network Respiratory Assist Device Guidelines respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. respiratory assist device (rad) qualifying guidelines. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. the information in this letter is intended to assist you in documenting that your. Respiratory Assist Device Guidelines.
From www.youtube.com
University of Pittsburgh Hemolung Respiratory Assist System YouTube Respiratory Assist Device Guidelines the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. a respiratory assist device. Respiratory Assist Device Guidelines.
From www.cayugamed.org
COVID19 Clinical Management Guidelines Cayuga Medical Center Respiratory Assist Device Guidelines the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. an abnormal. Respiratory Assist Device Guidelines.
From www.researchandmarkets.com
Respiratory Assist Devices Medical Devices Pipeline Product Landscape Respiratory Assist Device Guidelines a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. respiratory assist device (rad) qualifying guidelines. an. Respiratory Assist Device Guidelines.
From www.asthmafoundation.org.nz
Inhaler Devices Identification Chart Asthma Foundation NZ Respiratory Assist Device Guidelines the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. respiratory assist device (rad) qualifying guidelines. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. an. Respiratory Assist Device Guidelines.
From studylib.net
respiratory Minnesota Hospital Association Respiratory Assist Device Guidelines respiratory assist device (rad) qualifying guidelines. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30%. Respiratory Assist Device Guidelines.
From sleepandrespiratorydisorders.blogspot.com
Sleep and Respiratory Modalities How Respironics Trilogy 100 Helping Respiratory Assist Device Guidelines an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. the information in this letter is intended. Respiratory Assist Device Guidelines.
From healthylivinglinks.com
12 Devices To Help Pulmonary Rehabilitation And COPD Healthy Living Links Respiratory Assist Device Guidelines respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. a respiratory assist device (rad) without backup. Respiratory Assist Device Guidelines.
From rc.rcjournal.com
Pressure Support Ventilation Advisory System Provides Valid Respiratory Assist Device Guidelines a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. respiratory assist device (rad) qualifying guidelines. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria. Respiratory Assist Device Guidelines.
From healthandwillness.org
Oxygen Delivery Devices and Flow Rates Health And Willness Respiratory Assist Device Guidelines an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. respiratory assist devices include devices intended. Respiratory Assist Device Guidelines.
From www.ccs-sth.org
Critical Care Services STH Respiratory Physiotherapy Guidelines Respiratory Assist Device Guidelines an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) qualifying guidelines. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within. Respiratory Assist Device Guidelines.
From www.cmaj.ca
Diagnosis and management of acute respiratory distress syndrome CMAJ Respiratory Assist Device Guidelines the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. respiratory assist device (rad) qualifying guidelines. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. an. Respiratory Assist Device Guidelines.
From www.hcsgi.com
Respiratory Assist Devices Health Care Solutions Group Respiratory Assist Device Guidelines the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) qualifying guidelines. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. respiratory assist device (rad) documentation requirements for continued coverage beyond first. Respiratory Assist Device Guidelines.
From www.semanticscholar.org
Figure 1 from Study on Portable Respiratory Assist System for Respiratory Assist Device Guidelines respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. . Respiratory Assist Device Guidelines.
From www.nzrespiratoryguidelines.co.nz
Inhaler Identification NZ Respiratory Guidelines Respiratory Assist Device Guidelines respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) qualifying guidelines. respiratory assist device (rad) documentation requirements for continued coverage beyond first. Respiratory Assist Device Guidelines.
From www.researchgate.net
Respiratory therapist driven algorithm to guide airway clearance use Respiratory Assist Device Guidelines the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. an abnormal respiratory event. Respiratory Assist Device Guidelines.
From www.sleep.theclinics.com
Tailoring the Sleep Laboratory for Chronic Respiratory Failure Sleep Respiratory Assist Device Guidelines an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. the information in this letter is intended. Respiratory Assist Device Guidelines.
From allergyasthmanetwork.org
Respiratory Inhalers at a Glance and Other Posters in Our Online Store Respiratory Assist Device Guidelines an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist device (rad) qualifying guidelines.. Respiratory Assist Device Guidelines.
From www.prweb.com
TucsonBased Respiratory Device Company SaiOx Inc. Partners with India Respiratory Assist Device Guidelines a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. respiratory assist device (rad) qualifying guidelines. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. the information. Respiratory Assist Device Guidelines.
From www.helmetbasedventilation.com
inar NIV Respiratory Assist Helmets Evaluated by Emergency Care Respiratory Assist Device Guidelines respiratory assist device (rad) qualifying guidelines. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. respiratory assist devices include devices intended to help patients in need of support for breathing,. Respiratory Assist Device Guidelines.
From nursekey.com
Respiratory support noninvasive ventilation Nurse Key Respiratory Assist Device Guidelines respiratory assist device (rad) qualifying guidelines. respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. an abnormal respiratory event lasting at least 10 seconds associated with. Respiratory Assist Device Guidelines.
From hnbyond.en.made-in-china.com
High Flow Nasal Cannula Oxygen Therapy Oxygen Therapy Device with Respiratory Assist Device Guidelines an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. the information in this letter is intended to assist you in. Respiratory Assist Device Guidelines.
From www.cdc.gov
Elastomeric Respirator Resources NIOSH CDC Respiratory Assist Device Guidelines respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. respiratory assist device (rad) qualifying guidelines. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial.. Respiratory Assist Device Guidelines.
From www.hcsgi.com
Respiratory Assist Devices Health Care Solutions Group Respiratory Assist Device Guidelines respiratory assist device (rad) qualifying guidelines. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. the information in this. Respiratory Assist Device Guidelines.
From www.cpapman.com
Philips Respironics CoughAssist T70 Mask System Respiratory Assist Device Guidelines respiratory assist devices include devices intended to help patients in need of support for breathing, removal of carbon dioxide,. respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. an abnormal respiratory event lasting at least 10 seconds associated with at least a 30% reduction in. a respiratory assist device (rad) without backup. Respiratory Assist Device Guidelines.
From www.nsmedicaldevices.com
ALung gets FDA approval for Hemolung respiratory assist system Respiratory Assist Device Guidelines respiratory assist device (rad) documentation requirements for continued coverage beyond first 3 months. the information in this letter is intended to assist you in documenting that your patient meets medicare criteria for initial. a respiratory assist device (rad) without backup rate delivers adjustable, variable levels (within a single. respiratory assist device (rad) qualifying guidelines. an. Respiratory Assist Device Guidelines.