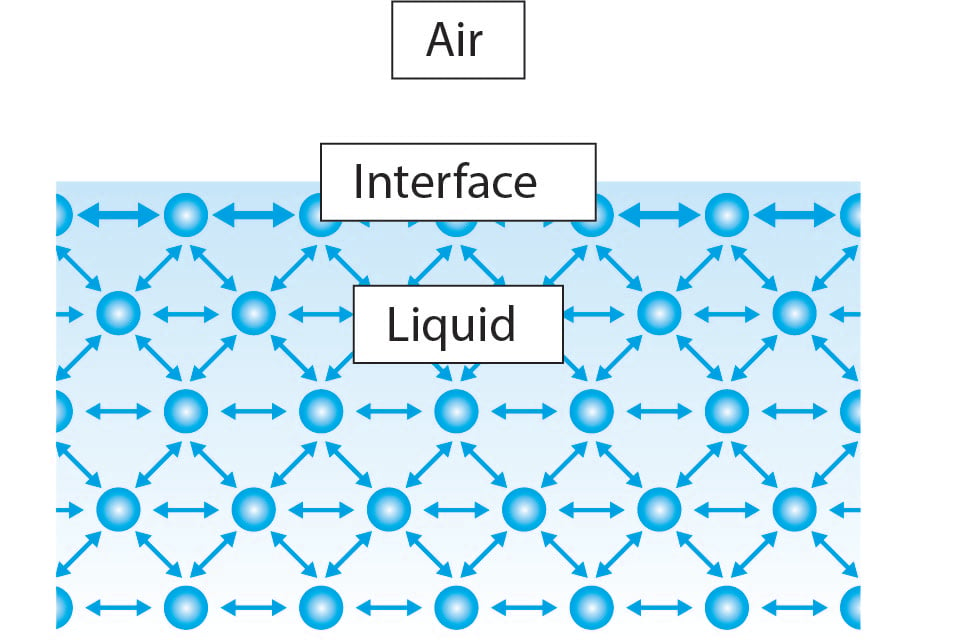
What Is Surface And Interfacial Tension . Typically mn/m (which is equivalent to. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. In this case it is called interfacial tension. — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line drawn in the surface. surface tension is the excess energy per unit area (force per unit length; when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another.
from www.biolinscientific.com
— the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line drawn in the surface. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. surface tension is the excess energy per unit area (force per unit length; when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. Typically mn/m (which is equivalent to. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. In this case it is called interfacial tension.
Glossary Knowledge Biolin Scientific
What Is Surface And Interfacial Tension the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line drawn in the surface. In this case it is called interfacial tension. Typically mn/m (which is equivalent to. when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. surface tension is the excess energy per unit area (force per unit length; — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Is Surface And Interfacial Tension — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation. What Is Surface And Interfacial Tension.
From www.slideserve.com
PPT Applied Chemistry PowerPoint Presentation, free download ID3528785 What Is Surface And Interfacial Tension surface tension is the excess energy per unit area (force per unit length; — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. — the interfacial tension. What Is Surface And Interfacial Tension.
From www.slideserve.com
PPT Retention, Stability & Support PowerPoint Presentation, free download ID1781221 What Is Surface And Interfacial Tension — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. the interfacial and surface tension of. What Is Surface And Interfacial Tension.
From www.slideshare.net
Interfacial phenomena What Is Surface And Interfacial Tension This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. In this case it is called interfacial tension. when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation. What Is Surface And Interfacial Tension.
From www.researchgate.net
Schematic illustration of equilibrium of three interfacial tensions (c... Download Scientific What Is Surface And Interfacial Tension the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line drawn in the surface. — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. In this case it is called interfacial tension. Typically. What Is Surface And Interfacial Tension.
From www.slideserve.com
PPT Surface and Interfacial Phenomena PowerPoint Presentation ID1597548 What Is Surface And Interfacial Tension when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. In this case it is called interfacial tension. Typically mn/m (which is equivalent to. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. the surface tension, γ, may be defined as the force/unit length parallel. What Is Surface And Interfacial Tension.
From www.youtube.com
Surface & Interfacial Tension , part2 (surface tension & its unit) YouTube What Is Surface And Interfacial Tension when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. In this case it is called interfacial tension. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. Typically mn/m (which is equivalent to. surface tension is the. What Is Surface And Interfacial Tension.
From www.slideshare.net
Surface and interfacial tension What Is Surface And Interfacial Tension — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. surface tension is the excess energy per unit area (force per unit length; This interfacial tension acts as. What Is Surface And Interfacial Tension.
From www.biolinscientific.com
Glossary Knowledge Biolin Scientific What Is Surface And Interfacial Tension — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension.. What Is Surface And Interfacial Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Is Surface And Interfacial Tension when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line. What Is Surface And Interfacial Tension.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Illustration of science What Is Surface And Interfacial Tension — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. — the same physics and chemistry that create surface tension also establish a distinct interface. What Is Surface And Interfacial Tension.
From www.youtube.com
Surface and interfacial Tension Introduction Liquid Interface L1Unit3 Physical P What Is Surface And Interfacial Tension when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. the interfacial. What Is Surface And Interfacial Tension.
From www.nanoscience.com
Surface and Interfacial Tension How, What and Why? Nanoscience Instruments What Is Surface And Interfacial Tension In this case it is called interfacial tension. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. when an interface forms between two immiscible liquids or two dissimilar. What Is Surface And Interfacial Tension.
From www.difference.wiki
Interfacial Tension vs. Surface Tension What’s the Difference? What Is Surface And Interfacial Tension when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. surface tension is the excess energy per unit area (force per unit length; — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. In this case it is called interfacial tension. . What Is Surface And Interfacial Tension.
From www.terrano.gr
Surface and Interfacial Tension What is It and How to Measure It Terrano What Is Surface And Interfacial Tension Typically mn/m (which is equivalent to. surface tension is the excess energy per unit area (force per unit length; when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. — the same physics and chemistry. What Is Surface And Interfacial Tension.
From www.researchgate.net
Surface tension, interfacial tension and oil contaminants solubility... Download Scientific What Is Surface And Interfacial Tension In this case it is called interfacial tension. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. Typically mn/m (which is equivalent to. surface tension is the excess energy per unit area (force per unit length; This interfacial tension acts as a barrier preventing one liquid from invading. What Is Surface And Interfacial Tension.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and Capillary Action What Is Surface And Interfacial Tension the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line drawn in the surface. when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another.. What Is Surface And Interfacial Tension.
From www.youtube.com
Difference between surface and interface surface tension and interfacial tension YouTube What Is Surface And Interfacial Tension — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. surface tension is the excess energy per unit area (force per unit length; Typically mn/m (which is equivalent to. In this case it is called interfacial tension. the surface tension, γ, may be defined as the force/unit length. What Is Surface And Interfacial Tension.
From www.scribd.com
Surface and Interfacial Tension What Is It and How To Measure It PDF Surface Tension Drop What Is Surface And Interfacial Tension — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. In this case it is called interfacial tension. the surface tension, γ, may be defined. What Is Surface And Interfacial Tension.
From www.scribd.com
Surface Tension and Interfacial Phenomenon PDF Surface Tension Adsorption What Is Surface And Interfacial Tension This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. In this case it is called interfacial tension. when an interface forms between two immiscible liquids or two dissimilar. What Is Surface And Interfacial Tension.
From www.slideshare.net
Surface and interfacial tension What Is Surface And Interfacial Tension Typically mn/m (which is equivalent to. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. surface tension is the excess energy per unit area (force per unit length; — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. when an interface. What Is Surface And Interfacial Tension.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL What Is Surface And Interfacial Tension In this case it is called interfacial tension. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line drawn in the surface. when an interface forms between two immiscible liquids. What Is Surface And Interfacial Tension.
From www.slideserve.com
PPT RETENTION, STABILITY & SUPPORT IN COMPLETE DENTURE PowerPoint Presentation ID9643894 What Is Surface And Interfacial Tension This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. Typically mn/m (which is equivalent to. — the interfacial tension is the surface free energy of the interface between. What Is Surface And Interfacial Tension.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it impart YouTube What Is Surface And Interfacial Tension — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. In this case it is called interfacial tension. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. Typically mn/m (which is equivalent to. when. What Is Surface And Interfacial Tension.
From www.youtube.com
Surface and Interfacial Tension (1/4) Physical Pharmacy GPAT NIPER BITS PCI B PHARMACY PHARMACAD What Is Surface And Interfacial Tension Typically mn/m (which is equivalent to. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. surface tension is the excess energy per unit area (force per unit length; the surface tension, γ, may be defined as the force/unit length parallel to the surface. What Is Surface And Interfacial Tension.
From byjus.com
Explain the surface tension phenomenon with examples. What Is Surface And Interfacial Tension — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids. — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. the surface tension, γ, may be defined as the force/unit length parallel to the surface. What Is Surface And Interfacial Tension.
From blog.biolinscientific.com
What is surface tension? What Is Surface And Interfacial Tension Typically mn/m (which is equivalent to. the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line drawn in the surface. In this case it is called interfacial tension. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a. What Is Surface And Interfacial Tension.
From www.slideshare.net
ppt SURFACE AND INTERFACIAL TENSION What Is Surface And Interfacial Tension the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line drawn in the surface. Typically mn/m (which is equivalent to. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. surface tension is the excess energy per unit area (force. What Is Surface And Interfacial Tension.
From dxoejuzoi.blob.core.windows.net
Surface And Interfacial Tension Measurement Theory And Applications at Emilio James blog What Is Surface And Interfacial Tension the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any line drawn in the surface. In this case it is called interfacial tension. . What Is Surface And Interfacial Tension.
From www.semanticscholar.org
Figure 1 from Measurement of surface and interfacial tension using pendant drop tensiometry What Is Surface And Interfacial Tension surface tension is the excess energy per unit area (force per unit length; the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. the surface tension, γ, may be defined as the force/unit length parallel to the surface which is exerted perpendicular to any. What Is Surface And Interfacial Tension.
From www.slideserve.com
PPT Retention, Stability & Support PowerPoint Presentation, free download ID1781221 What Is Surface And Interfacial Tension the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. surface tension is the excess energy per unit area (force per unit length; Typically mn/m (which is equivalent to. — the same physics and chemistry that create surface tension also establish a distinct interface. What Is Surface And Interfacial Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Is Surface And Interfacial Tension — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. — the interfacial tension is the surface free energy of the interface between two immiscible, or poorly miscible liquids.. What Is Surface And Interfacial Tension.
From www.askdifference.com
Interfacial Tension vs. Surface Tension — What’s the Difference? What Is Surface And Interfacial Tension when an interface forms between two immiscible liquids or two dissimilar phases, a similar situation occurs. — the same physics and chemistry that create surface tension also establish a distinct interface between two immiscible liquids, leading to interfacial tension. This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. Typically mn/m (which. What Is Surface And Interfacial Tension.
From www.youtube.com
SURFACE TENSION & INTERFACIAL PHENOMENON PART1 INTERFACE TYPES OF INTERFACE IMPORTANCE What Is Surface And Interfacial Tension This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. surface tension is the excess energy per unit area (force per unit length; the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. In this case it is called. What Is Surface And Interfacial Tension.
From www.kruss-scientific.com
Interfacial tension KRÜSS Scientific What Is Surface And Interfacial Tension This interfacial tension acts as a barrier preventing one liquid from invading the surface of another. the interfacial and surface tension of a liquid allows conclusions about how well the liquid spreads on a solid or mixes with another. surface tension is the excess energy per unit area (force per unit length; In this case it is called. What Is Surface And Interfacial Tension.