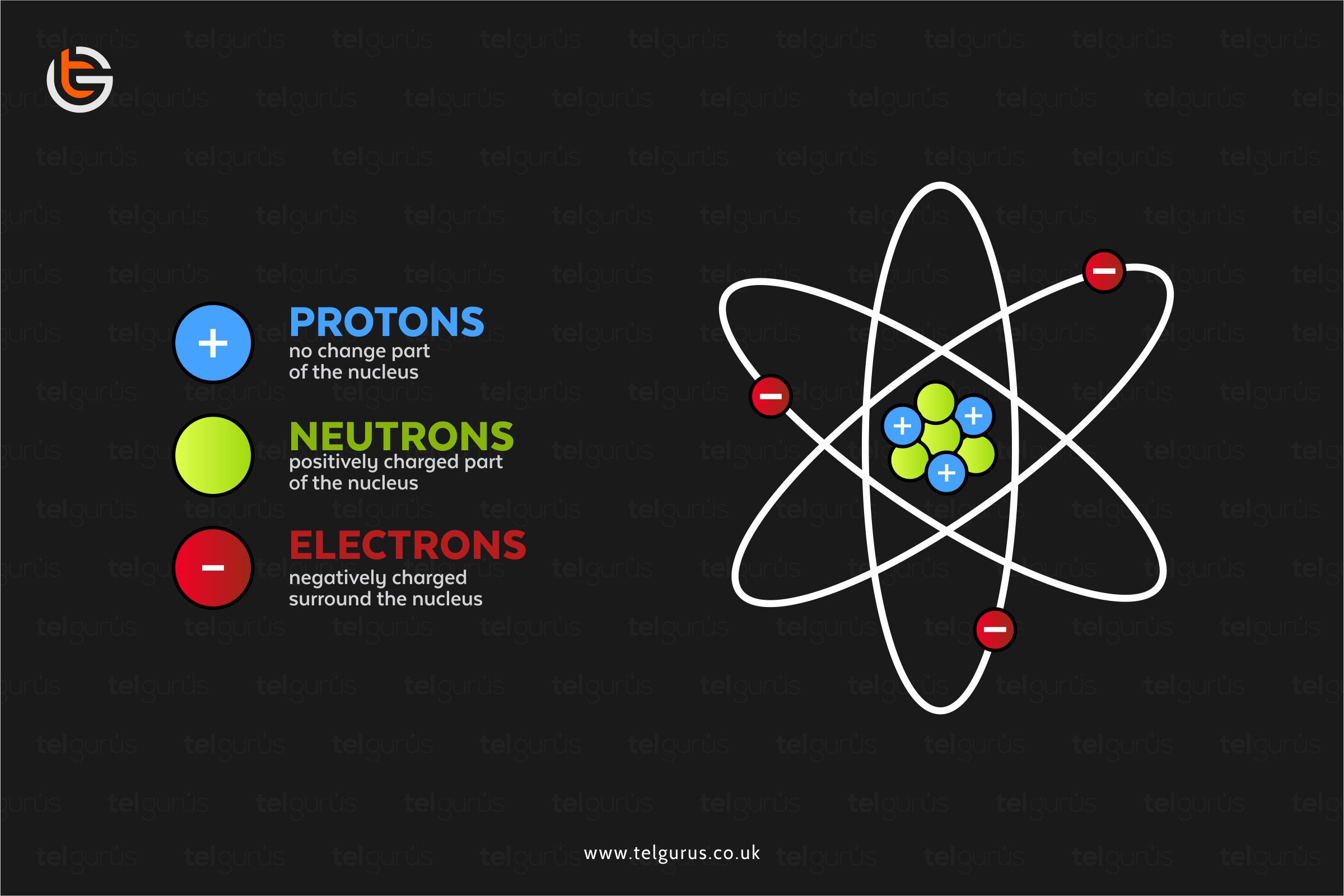
Do All Atoms Have A Positive Charge . The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Let's take a look at charge first. In a neutral atom, there are equal numbers of protons and. Protons have a positive charge of one plus, and electrons have a negative charge of one minus. A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Together with neutrons, they make up virtually all of the mass of an atom. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),.
from telgurus.co.uk
Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Let's take a look at charge first. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. In a neutral atom, there are equal numbers of protons and. Protons have a positive charge of one plus, and electrons have a negative charge of one minus. Together with neutrons, they make up virtually all of the mass of an atom.
What is the overall charge of the nucleus of an atom and why?
Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Protons have a positive charge of one plus, and electrons have a negative charge of one minus. In a neutral atom, there are equal numbers of protons and. Together with neutrons, they make up virtually all of the mass of an atom. Let's take a look at charge first. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu).
From www.slideserve.com
PPT Lecture 1 Charge and Coulomb’s Law PowerPoint Presentation, free Do All Atoms Have A Positive Charge In a neutral atom, there are equal numbers of protons and. Let's take a look at charge first. Protons have a positive charge of one plus, and electrons have a negative charge of one minus. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Protons have a positive electrical. Do All Atoms Have A Positive Charge.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Do All Atoms Have A Positive Charge Protons have a positive charge of one plus, and electrons have a negative charge of one minus. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu}. Do All Atoms Have A Positive Charge.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Protons have a positive charge of one plus, and electrons have a negative charge of one minus. Together. Do All Atoms Have A Positive Charge.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Do All Atoms Have A Positive Charge A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Together with neutrons, they make up virtually all of the mass of an atom. Protons have a positive charge of. Do All Atoms Have A Positive Charge.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is.. Do All Atoms Have A Positive Charge.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Do All Atoms Have A Positive Charge A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Together with neutrons, they make up virtually all of the mass of an atom. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Protons have a positive electrical charge of one. Do All Atoms Have A Positive Charge.
From www.pinterest.com
Spectroscopy Electron configuration, Chemistry education, Protons Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive charge of one plus, and electrons have a negative charge of one. Do All Atoms Have A Positive Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Do All Atoms Have A Positive Charge The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit. Do All Atoms Have A Positive Charge.
From www.slideserve.com
PPT Protons are positive (+) charged particles found in the nucleus Do All Atoms Have A Positive Charge Together with neutrons, they make up virtually all of the mass of an atom. Let's take a look at charge first. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Protons have a positive charge of one plus, and electrons have a negative charge of one minus. Protons have. Do All Atoms Have A Positive Charge.
From www.examanalysis.in
Electric Current Do All Atoms Have A Positive Charge Protons have a positive charge of one plus, and electrons have a negative charge of one minus. In a neutral atom, there are equal numbers of protons and. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Together with neutrons, they make up virtually all of the. Do All Atoms Have A Positive Charge.
From www.expii.com
Atoms — Definition & Overview Expii Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). In a neutral atom, there are equal numbers of protons and. A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Protons have a positive charge of one plus, and electrons have. Do All Atoms Have A Positive Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Do All Atoms Have A Positive Charge Let's take a look at charge first. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive charge of one plus, and. Do All Atoms Have A Positive Charge.
From slideplayer.com
Understanding Electrons ppt download Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Let's take a look at charge first. The single most important characteristic of an atom is its atomic number (usually. Do All Atoms Have A Positive Charge.
From sphweb.bumc.bu.edu
Chemical Elements Atoms Do All Atoms Have A Positive Charge A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left(. Do All Atoms Have A Positive Charge.
From slideplayer.com
Atomic structure Mrs. Meadows. ppt download Do All Atoms Have A Positive Charge Protons have a positive charge of one plus, and electrons have a negative charge of one minus. A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. The single most. Do All Atoms Have A Positive Charge.
From diagramliboriginariosmb1.z13.web.core.windows.net
Diagram Of Structure Of Atom Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Together with neutrons, they make up virtually all of the mass of an atom. A proton carries a. Do All Atoms Have A Positive Charge.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology Do All Atoms Have A Positive Charge The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Together with neutrons, they make up virtually all of the mass of an atom. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Let's take a look. Do All Atoms Have A Positive Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Do All Atoms Have A Positive Charge Protons have a positive charge of one plus, and electrons have a negative charge of one minus. In a neutral atom, there are equal numbers of protons and. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. A proton carries a positive electrical charge, a neutron is. Do All Atoms Have A Positive Charge.
From www.mindomo.com
Energy Mind Map Do All Atoms Have A Positive Charge A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Let's take a look at charge first. In a neutral atom, there are equal numbers of protons and. The single most important. Do All Atoms Have A Positive Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). The single most important characteristic of an atom is its atomic number (usually denoted by the letter z),. Do All Atoms Have A Positive Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Together with neutrons, they make up virtually all of the mass of an atom. Let's take a look. Do All Atoms Have A Positive Charge.
From calebcroomphysci4dummies.weebly.com
Atomic Structure Physical Science For Dummies Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu).. Do All Atoms Have A Positive Charge.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry Do All Atoms Have A Positive Charge Together with neutrons, they make up virtually all of the mass of an atom. In a neutral atom, there are equal numbers of protons and. Let's take a look at charge first. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge. Do All Atoms Have A Positive Charge.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview Do All Atoms Have A Positive Charge The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. In a neutral atom, there are equal numbers of protons and. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Let's take a look at charge first. Together with. Do All Atoms Have A Positive Charge.
From www.masterorganicchemistry.com
7 Factors That Stabilize Positive Charge in Organic Chemistry Master Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive charge of one plus, and electrons have a negative charge of one minus. In a neutral atom, there are equal numbers of protons and. Let's take a look at charge first. Protons have a. Do All Atoms Have A Positive Charge.
From sciencenotes.org
Learn the Parts of an Atom Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Together with neutrons, they make up virtually all of the mass of an atom. In a neutral atom,. Do All Atoms Have A Positive Charge.
From slideplayer.com
Ions and Ionic Compounds ppt download Do All Atoms Have A Positive Charge Let's take a look at charge first. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive charge of one plus, and electrons have a negative charge of one minus. In a neutral atom, there are equal numbers of protons and. Protons have a. Do All Atoms Have A Positive Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Let's take a look at charge first. The single most important characteristic of an atom is its atomic number (usually. Do All Atoms Have A Positive Charge.
From studyrocket.co.uk
The Structure of the Atom GCSE Physics Science) AQA Do All Atoms Have A Positive Charge Protons have a positive charge of one plus, and electrons have a negative charge of one minus. Together with neutrons, they make up virtually all of the mass of an atom. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of. Do All Atoms Have A Positive Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Do All Atoms Have A Positive Charge In a neutral atom, there are equal numbers of protons and. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Let's take a look at charge first. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),.. Do All Atoms Have A Positive Charge.
From www.sliderbase.com
Atoms, molecules and ions Presentation Chemistry Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),.. Do All Atoms Have A Positive Charge.
From telgurus.co.uk
What is the overall charge of the nucleus of an atom and why? Do All Atoms Have A Positive Charge Protons have a positive charge of one plus, and electrons have a negative charge of one minus. The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. A. Do All Atoms Have A Positive Charge.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Do All Atoms Have A Positive Charge Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Let's take a look at charge first. Protons have a positive charge of one plus, and electrons have. Do All Atoms Have A Positive Charge.
From www.slideserve.com
PPT What part of an atom is the arrow pointing to? PowerPoint Do All Atoms Have A Positive Charge Together with neutrons, they make up virtually all of the mass of an atom. A proton carries a positive electrical charge, a neutron is neutral, and an electron has a negative charge. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. The single most important characteristic of. Do All Atoms Have A Positive Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Dynamic Periodic Do All Atoms Have A Positive Charge Together with neutrons, they make up virtually all of the mass of an atom. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). The single most important characteristic of an atom is its atomic number (usually denoted by the letter z), which is. A proton carries a positive electrical. Do All Atoms Have A Positive Charge.