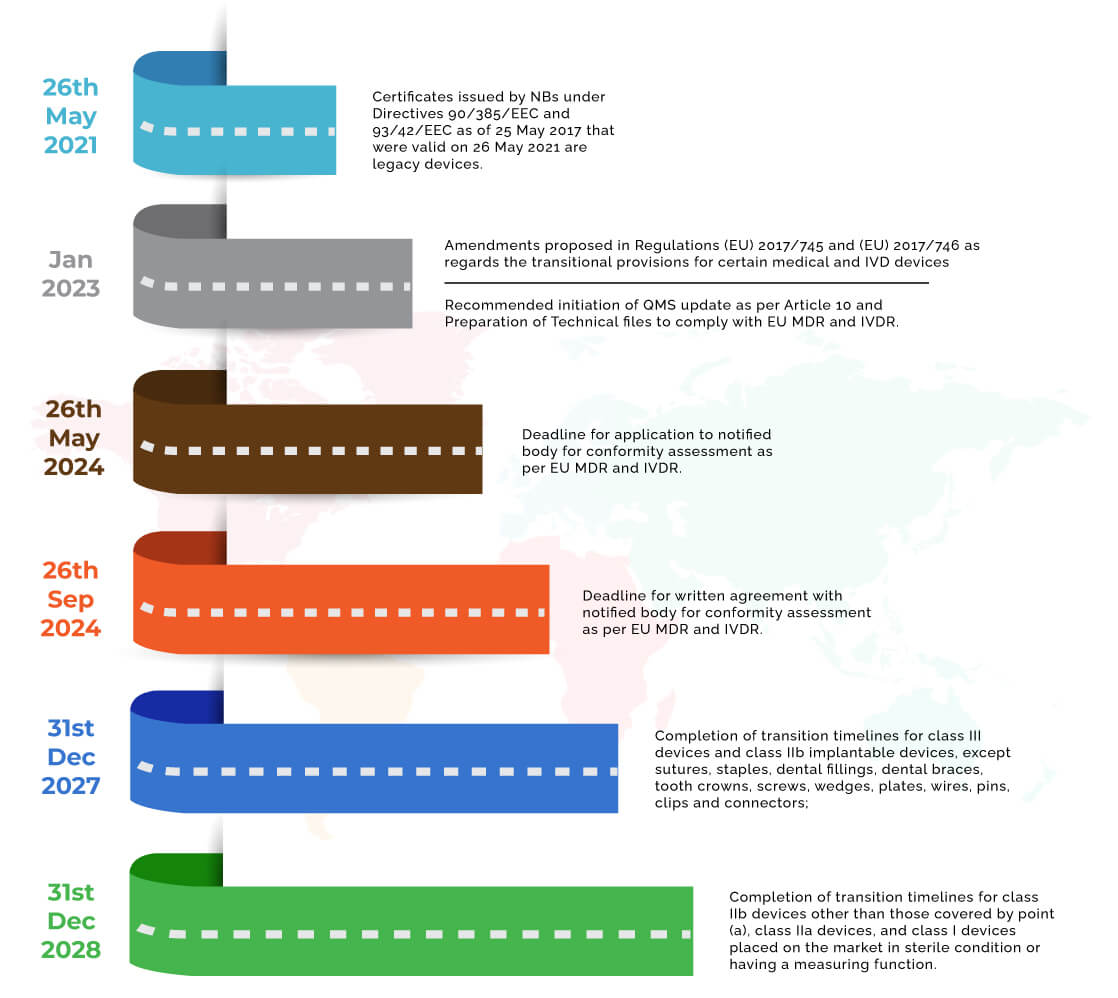
Medical Device Regulations Eu Mdr . The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. G) established by article 103 of regulation (eu) 2017/745. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation applies since 26 may 2021. Manufacturers must comply with the regulation when placing new medical devices. Guidance on classification of medical devices. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746.
from www.vrogue.co
Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation applies since 26 may 2021. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. G) established by article 103 of regulation (eu) 2017/745. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Manufacturers must comply with the regulation when placing new medical devices. Guidance on classification of medical devices.
Eu Mdr Medical Device Regulations Timeline vrogue.co
Medical Device Regulations Eu Mdr Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. G) established by article 103 of regulation (eu) 2017/745. The medical devices regulation applies since 26 may 2021. Guidance on classification of medical devices. Manufacturers must comply with the regulation when placing new medical devices. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.
From medrio.com
European MDR (EU MDR) Guide to Prepare Medical Device Regulations Eu Mdr The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Guidance on classification of medical devices. Manufacturers must comply with the regulation when placing new medical devices. The medical devices regulation applies since 26 may 2021. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Medical Device Regulations Eu Mdr.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Regulations Eu Mdr G) established by article 103 of regulation (eu) 2017/745. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Guidance on classification of medical devices. Manufacturers must comply with the regulation when placing new medical devices. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745. Medical Device Regulations Eu Mdr.
From www.shutterstock.com
Mdr Medical Device Regulation Regulation Eu Stock Vector (Royalty Free Medical Device Regulations Eu Mdr The medical devices regulation applies since 26 may 2021. Manufacturers must comply with the regulation when placing new medical devices. Guidance on classification of medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Proposal for a regulation of the european parliament and of the council amending regulations. Medical Device Regulations Eu Mdr.
From www.jamasoftware.com
EU MDR FAQs Industry Expert Insights Jama Software Medical Device Regulations Eu Mdr Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical devices regulation applies since 26 may 2021. Guidance on classification of medical devices. Proposal for a regulation of the european. Medical Device Regulations Eu Mdr.
From medrio.com
EU MDR European Medical Device Regulation Explained Medical Device Regulations Eu Mdr The medical devices regulation applies since 26 may 2021. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Guidance on classification of medical devices. G) established by article 103 of regulation. Medical Device Regulations Eu Mdr.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I Medical Device Regulations Eu Mdr The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Guidance on classification of medical devices. Manufacturers must comply with the regulation when placing new medical devices. The medical devices regulation applies since 26 may 2021. G) established by article 103 of regulation (eu) 2017/745. Regulation (eu) 2017/745 of the european parliament. Medical Device Regulations Eu Mdr.
From crfweb.com
Medical Device Regulations Medical Device Regulations Eu Mdr Guidance on classification of medical devices. The medical devices regulation applies since 26 may 2021. Manufacturers must comply with the regulation when placing new medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Proposal for a regulation of the european parliament and of the council amending regulations. Medical Device Regulations Eu Mdr.
From www.jamasoftware.com
What the New Medical Device Regulations (EU MDR) Mean for You Jama Medical Device Regulations Eu Mdr The medical devices regulation applies since 26 may 2021. Manufacturers must comply with the regulation when placing new medical devices. G) established by article 103 of regulation (eu) 2017/745. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Guidance on classification of medical devices. Regulation (eu) 2017/745 of the european parliament. Medical Device Regulations Eu Mdr.
From www.mastercontrol.com
Q&A Device Manufacturers’ FAQs on the EU’s Medical Device Regulation (MDR) Medical Device Regulations Eu Mdr Manufacturers must comply with the regulation when placing new medical devices. G) established by article 103 of regulation (eu) 2017/745. Guidance on classification of medical devices. The medical devices regulation applies since 26 may 2021. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. The medical device regulation (mdr), which. Medical Device Regulations Eu Mdr.
From www.linkedin.com
Medical Device Regulation MDR will apply from May 26, 2021 Medical Device Regulations Eu Mdr Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Guidance on classification. Medical Device Regulations Eu Mdr.
From luzernbaar.ch
Are you ready for the MDR? This is how new EU regulations may impact Medical Device Regulations Eu Mdr G) established by article 103 of regulation (eu) 2017/745. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Manufacturers must comply with the regulation when placing new medical devices. The medical devices regulation applies since 26 may 2021. Guidance on classification of medical devices. The medical device regulation (mdr), which. Medical Device Regulations Eu Mdr.
From gxp-training.com
Medical Device Regulation MDR 2017/745 Course and Certificate Medical Device Regulations Eu Mdr Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical devices regulation applies since 26 may 2021. Guidance on classification of medical devices. Proposal for a regulation of the european. Medical Device Regulations Eu Mdr.
From www.tuv.com
EU Medical Device Regulation MDR 2017/745 AM TÜV Rheinland Medical Device Regulations Eu Mdr G) established by article 103 of regulation (eu) 2017/745. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Manufacturers must comply with the regulation when placing new medical devices. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Proposal for. Medical Device Regulations Eu Mdr.
From www.presentationeze.com
MDR Medical Device Regulation EU 2017 745 Timeline PresentationEZE Medical Device Regulations Eu Mdr Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Guidance on classification of medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal. Medical Device Regulations Eu Mdr.
From www.lek.com
European Medical Devices Regulations and Their Impact Medical Device Regulations Eu Mdr The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Manufacturers must comply with the regulation when placing new medical devices. The medical devices regulation applies since 26 may 2021. G) established by article 103 of regulation (eu) 2017/745. Regulation (eu) 2017/745 of the european parliament and of the council of 5. Medical Device Regulations Eu Mdr.
From pharmait.dk
Concept, Of, Mdr, Medical, Device, Regulation. Pharma IT Medical Device Regulations Eu Mdr Guidance on classification of medical devices. Manufacturers must comply with the regulation when placing new medical devices. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. G) established by article. Medical Device Regulations Eu Mdr.
From www.iascertification.com
EUMDR Certification Medical Device Regulation IAS Medical Device Regulations Eu Mdr The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Manufacturers must comply with the regulation when placing new medical devices. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Guidance on classification of medical devices. Regulation (eu) 2017/745 of the european. Medical Device Regulations Eu Mdr.
From www.kolabtree.com
Prepare your medical device for EU MDR 8 trusted resources Medical Device Regulations Eu Mdr G) established by article 103 of regulation (eu) 2017/745. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Manufacturers must comply with the regulation when placing new medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The. Medical Device Regulations Eu Mdr.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Medical Device Regulations Eu Mdr The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. G) established by article 103 of regulation (eu) 2017/745. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. The medical devices regulation applies since 26 may 2021. Manufacturers must comply with the. Medical Device Regulations Eu Mdr.
From connectorsupplier.com
EU MDR Update to Medical Device Regulations in Europe Medical Device Regulations Eu Mdr The medical devices regulation applies since 26 may 2021. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Manufacturers must comply with the regulation when placing new medical devices. Proposal for. Medical Device Regulations Eu Mdr.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Medical Device Regulations Eu Mdr Manufacturers must comply with the regulation when placing new medical devices. Guidance on classification of medical devices. The medical devices regulation applies since 26 may 2021. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. G) established by article 103 of regulation (eu) 2017/745. Proposal for a regulation of the european. Medical Device Regulations Eu Mdr.
From www.greenlight.guru
Ultimate Guide to Device Class Requirements under EU MDR Medical Device Regulations Eu Mdr Manufacturers must comply with the regulation when placing new medical devices. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical devices regulation applies since 26 may 2021. G) established by. Medical Device Regulations Eu Mdr.
From easymedicaldevice.com
EU MDR 2017/745 Transition timeline [Medical Device Regulation] Medical Device Regulations Eu Mdr Guidance on classification of medical devices. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical. Medical Device Regulations Eu Mdr.
From www.castoredc.com
Overview of EU Medical Device Regulations (MDR) Medical Device Regulations Eu Mdr The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Manufacturers must comply with the regulation when placing new medical devices. Guidance on classification of medical devices. G) established by article 103 of regulation (eu) 2017/745. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Medical Device Regulations Eu Mdr.
From operonstrategist.com
Comprehensive Guide to the EU Medical Device Regulation (EU MDR Medical Device Regulations Eu Mdr G) established by article 103 of regulation (eu) 2017/745. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. The medical devices regulation applies since 26 may 2021. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Guidance on classification. Medical Device Regulations Eu Mdr.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Medical Device Regulations Eu Mdr G) established by article 103 of regulation (eu) 2017/745. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Proposal for a regulation of the european parliament and of the council amending. Medical Device Regulations Eu Mdr.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU Medical Device Regulations Eu Mdr The medical devices regulation applies since 26 may 2021. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april. Medical Device Regulations Eu Mdr.
From www.vrogue.co
Eu Mdr Medical Device Regulations Timeline vrogue.co Medical Device Regulations Eu Mdr G) established by article 103 of regulation (eu) 2017/745. Guidance on classification of medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation applies since 26 may 2021. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745. Medical Device Regulations Eu Mdr.
From www.vrogue.co
Eu Mdr Medical Device Regulations Timeline vrogue.co Medical Device Regulations Eu Mdr Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Guidance on classification of medical devices. The medical devices regulation applies since 26 may 2021. Manufacturers must comply with the regulation when placing new medical devices. G) established by article 103 of regulation (eu) 2017/745. Proposal for a regulation of. Medical Device Regulations Eu Mdr.
From www.greenlight.guru
The Medical Device Practical Guide to PMCF Requirements under EU MDR Medical Device Regulations Eu Mdr The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. G) established by article 103 of regulation (eu) 2017/745. The medical devices regulation applies since 26 may 2021. Regulation (eu) 2017/745 of the. Medical Device Regulations Eu Mdr.
From www.regulatoryglobe.com
EU MDR implementation guide for medical devices MDCG Medical Device Regulations Eu Mdr G) established by article 103 of regulation (eu) 2017/745. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. The medical devices regulation applies since 26 may 2021. The medical device. Medical Device Regulations Eu Mdr.
From blog.cosmotrace.com
Medical Devices Regulations (MDR) Medical Device Regulations Eu Mdr The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. G) established by article 103 of regulation (eu) 2017/745. Guidance on classification of medical devices. The medical devices regulation applies since 26 may 2021. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu). Medical Device Regulations Eu Mdr.
From gbu-taganskij.ru
EU MDR Medical Device Labeling Changes And Challenges By, 47 OFF Medical Device Regulations Eu Mdr The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Guidance on classification. Medical Device Regulations Eu Mdr.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Regulations Eu Mdr Proposal for a regulation of the european parliament and of the council amending regulations (eu) 2017/745 and (eu) 2017/746. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. G) established by article 103 of regulation (eu) 2017/745. The medical device regulation (mdr), which was adopted in april 2017, changes. Medical Device Regulations Eu Mdr.
From www.universalmedica.com
Key Aspects of New EU Medical Devices Regulation (EU 2017/745 Medical Device Regulations Eu Mdr Guidance on classification of medical devices. Manufacturers must comply with the regulation when placing new medical devices. G) established by article 103 of regulation (eu) 2017/745. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Proposal for a regulation of the european parliament and of the council amending regulations. Medical Device Regulations Eu Mdr.