Matrix Medical Devices . A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states.
from www.slideserve.com
when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. A traceability matrix is also an excellent internal tool for project management purposes.
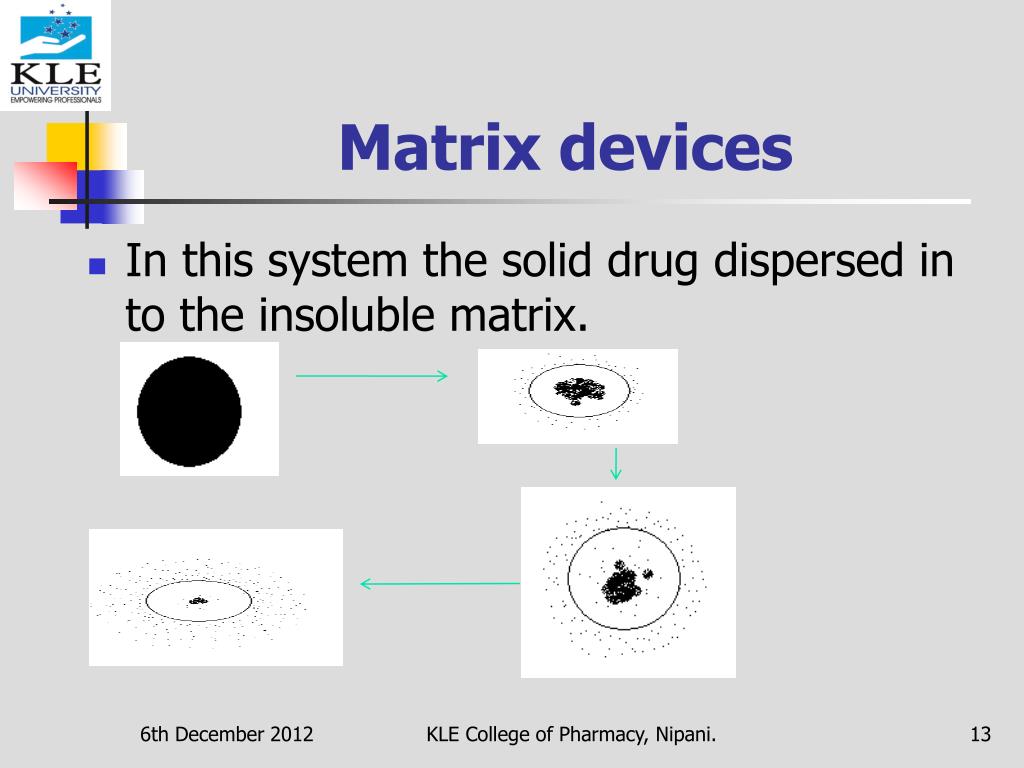
PPT Controlled Release Oral Drug Delivery System PowerPoint
Matrix Medical Devices when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an excellent internal tool for project management purposes.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From www.perforce.com
Condensed Guide to Medical Device Requirements Management Perforce Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste. Matrix Medical Devices.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an. Matrix Medical Devices.
From mavink.com
Traceability Matrix Medical Device Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From emmainternational.com
Importance of Traceability Matrix for Medical Devices Matrix Medical Devices when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste. Matrix Medical Devices.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an. Matrix Medical Devices.
From www.ce-marking.com
Guide on Class IIb MDD Medical Devices CE marking (mark) & European Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an. Matrix Medical Devices.
From www.linkedin.com
Traceability Matrix Medical Device Test for Success Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From www.matrix-innovations.com
MATRIX Program MATRIX GmbH CLAMPING TECHNOLOGY Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an. Matrix Medical Devices.
From www.glassdoor.com
Working at Matrix Medical Network Glassdoor Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From www.slideserve.com
PPT Controlled Release Oral Drug Delivery System PowerPoint Matrix Medical Devices when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste. Matrix Medical Devices.
From www.velosio.com
Medical Device Field Service Velosio Matrix Medical Devices when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. A traceability matrix is also an. Matrix Medical Devices.
From 3dmatrix.com
3D Matrix medical devices based on selfassembling peptide technology Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From exoaernau.blob.core.windows.net
Medical Device Risk Management Documents at Terrence Knapp blog Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste. Matrix Medical Devices.
From hawkequip.com
MATRIX Hawk Equipment Services Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste. Matrix Medical Devices.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Matrix Medical Devices when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste. Matrix Medical Devices.
From www.amazon.in
Buy Matrix Medical IMAK SmartGlove Wrist Support, Smart Glv Md, (1 EACH Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From dribbble.com
Matrix Medical Devices Logo by Codesummit on Dribbble Matrix Medical Devices when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste. Matrix Medical Devices.
From simbex.com
How to Define Product Requirements for Medical Devices Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From www.prnewswire.com
Matrix Medical Network Announces Sale of Decentralized Clinical Trials Matrix Medical Devices when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. A traceability matrix is also an. Matrix Medical Devices.
From www.orielstat.com
Basics of Medical Device Design Controls What, Why, and How Oriel Matrix Medical Devices when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste. Matrix Medical Devices.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From collagenmd.guna.com
MDMatrix Guna collagen medical devices Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste. Matrix Medical Devices.
From azbigmedia.com
Scottsdale's Matrix Medical Network plays key role in COVID19 vaccine Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an. Matrix Medical Devices.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.
From medicaldevicehq.com
Performing medical device risk evaluation Medical Device HQ Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste. Matrix Medical Devices.
From www.crainscleveland.com
Matrix Medical Devices of Cleveland purchases Mentorbased REU Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste. Matrix Medical Devices.
From shop.inomed.com
inomed Medizintechnik GmbH MEP Stimulation Adaptor for Grid/TES Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste. Matrix Medical Devices.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. 5900 landerbrook dr ste. Matrix Medical Devices.
From my.clevelandclinic.org
Incubator Clients Partner Cleveland Clinic Innovations Matrix Medical Devices 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an. Matrix Medical Devices.
From www.modernrequirements.com
Facilitate the Medical Device Design Controls Modern Requirements Matrix Medical Devices when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the goals you have set have been met. A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste. Matrix Medical Devices.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Matrix Medical Devices A traceability matrix is also an excellent internal tool for project management purposes. 5900 landerbrook dr ste 350, mayfield heights, ohio, 44124, united states. when done effectively, a traceability matrix will allow a medical device manufacturer to see a clear path between the resulting physical product and the design history, including evidence of quality controls and that the. Matrix Medical Devices.