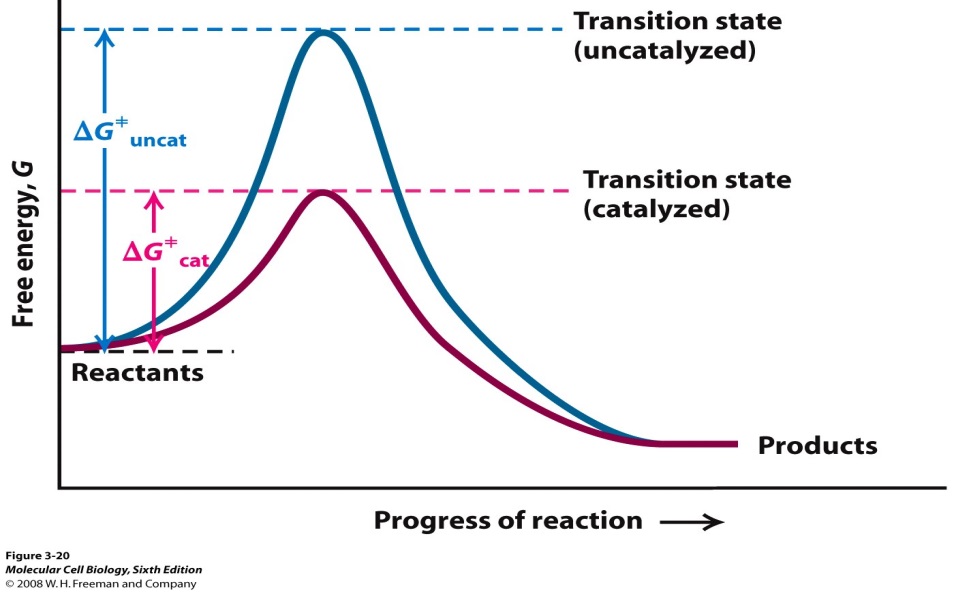
A Catalyst Can Increase The Rate Of A Reaction By . A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. this change is brought about by a specific interaction between the catalyst and the reaction components. However, not all reactions have. the rate of a reaction can be increased by adding a suitable catalyst. this page explains how adding a catalyst affects the rate of a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst does not actually. A catalyst is a substance which increases. only a very small mass of catalyst is needed to increase the rate of a reaction. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. Catalysts can be homogenous (in the same. It assumes that you are already familiar with basic ideas about the. this page describes and explains the way that adding a catalyst affects the rate of a reaction.
from exoqpbamm.blob.core.windows.net
Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. A catalyst is a substance which increases. the rate of a reaction can be increased by adding a suitable catalyst. this change is brought about by a specific interaction between the catalyst and the reaction components. only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic ideas about the. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Catalysts can be homogenous (in the same. A catalyst does not actually. this page explains how adding a catalyst affects the rate of a reaction.
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at
A Catalyst Can Increase The Rate Of A Reaction By this page describes and explains the way that adding a catalyst affects the rate of a reaction. only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have. It assumes that you are already familiar with basic ideas about the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same. this page describes and explains the way that adding a catalyst affects the rate of a reaction. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. A catalyst does not actually. the rate of a reaction can be increased by adding a suitable catalyst. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. this change is brought about by a specific interaction between the catalyst and the reaction components. A catalyst is a substance which increases. this page explains how adding a catalyst affects the rate of a reaction.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at A Catalyst Can Increase The Rate Of A Reaction By only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic ideas about the. A catalyst does not actually. the rate of a reaction can be increased by adding a suitable catalyst. this page explains how adding a catalyst affects the rate of. A Catalyst Can Increase The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Catalyst Can Increase The Rate Of A Reaction By catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same. this change is brought about by a specific interaction between the catalyst and the reaction components. However, not all reactions have. A catalyst is a substance that can help the reactants in a. A Catalyst Can Increase The Rate Of A Reaction By.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical A Catalyst Can Increase The Rate Of A Reaction By catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It assumes that you are already familiar with basic ideas about the. this page explains how adding a catalyst affects the rate of a reaction. Catalysts can be homogenous (in the same. A catalyst is a substance that can help. A Catalyst Can Increase The Rate Of A Reaction By.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry A Catalyst Can Increase The Rate Of A Reaction By However, not all reactions have. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. this change is brought about by a specific interaction between the catalyst and the reaction components. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy.. A Catalyst Can Increase The Rate Of A Reaction By.
From www.slideshare.net
Rate of reaction(f5) A Catalyst Can Increase The Rate Of A Reaction By this page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. only a very small mass of catalyst is needed to increase the rate of a reaction. catalysts affect the rate of. A Catalyst Can Increase The Rate Of A Reaction By.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 A Catalyst Can Increase The Rate Of A Reaction By catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. this change is brought about by a specific interaction between the catalyst and the reaction components. However, not all reactions have. this page describes and explains the way that adding a catalyst affects the rate of a reaction. It. A Catalyst Can Increase The Rate Of A Reaction By.
From www.youtube.com
Factors Affecting the Rate of Reaction Catalyst Rate of Reaction A Catalyst Can Increase The Rate Of A Reaction By It assumes that you are already familiar with basic ideas about the. the rate of a reaction can be increased by adding a suitable catalyst. A catalyst does not actually. this page explains how adding a catalyst affects the rate of a reaction. this change is brought about by a specific interaction between the catalyst and the. A Catalyst Can Increase The Rate Of A Reaction By.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID A Catalyst Can Increase The Rate Of A Reaction By this change is brought about by a specific interaction between the catalyst and the reaction components. only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst does not actually. this page describes and explains the way that adding a catalyst affects the rate of a reaction. the rate. A Catalyst Can Increase The Rate Of A Reaction By.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the A Catalyst Can Increase The Rate Of A Reaction By the rate of a reaction can be increased by adding a suitable catalyst. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts affect the rate of a chemical reaction. A Catalyst Can Increase The Rate Of A Reaction By.
From www.numerade.com
SOLVED A catalyst can increase the rate of a reaction by?The A Catalyst Can Increase The Rate Of A Reaction By Catalysts can be homogenous (in the same. this page describes and explains the way that adding a catalyst affects the rate of a reaction. this change is brought about by a specific interaction between the catalyst and the reaction components. A catalyst does not actually. Is a substance that increases the rate of reaction, but can be recovered,. A Catalyst Can Increase The Rate Of A Reaction By.
From dxorowdpy.blob.core.windows.net
Do Catalysts Increase The Concentration Of Reactants at Daniel Glover blog A Catalyst Can Increase The Rate Of A Reaction By A catalyst does not actually. the rate of a reaction can be increased by adding a suitable catalyst. only a very small mass of catalyst is needed to increase the rate of a reaction. this page explains how adding a catalyst affects the rate of a reaction. this change is brought about by a specific interaction. A Catalyst Can Increase The Rate Of A Reaction By.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii A Catalyst Can Increase The Rate Of A Reaction By Catalysts can be homogenous (in the same. A catalyst does not actually. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. this page explains how adding a catalyst affects the rate of a reaction. this change is brought about by a specific interaction between the catalyst and the reaction components.. A Catalyst Can Increase The Rate Of A Reaction By.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) A Catalyst Can Increase The Rate Of A Reaction By this change is brought about by a specific interaction between the catalyst and the reaction components. Catalysts can be homogenous (in the same. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. this page explains how adding a catalyst affects the rate of a reaction. A catalyst does not actually.. A Catalyst Can Increase The Rate Of A Reaction By.
From www.chegg.com
Solved A catalyst can increase the rate of a reaction by A Catalyst Can Increase The Rate Of A Reaction By Catalysts can be homogenous (in the same. A catalyst is a substance which increases. the rate of a reaction can be increased by adding a suitable catalyst. A catalyst does not actually. this change is brought about by a specific interaction between the catalyst and the reaction components. It assumes that you are already familiar with basic ideas. A Catalyst Can Increase The Rate Of A Reaction By.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction A Catalyst Can Increase The Rate Of A Reaction By A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst does not actually. A catalyst is a substance which increases. Catalysts can be homogenous (in the same. However, not all reactions have. the rate of a reaction can be increased by adding a suitable catalyst. only a. A Catalyst Can Increase The Rate Of A Reaction By.
From www.numerade.com
⏩SOLVEDWhy does a catalyst increase the rate of a reaction? What is A Catalyst Can Increase The Rate Of A Reaction By catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst does not actually. It assumes that you are already familiar with basic ideas about the. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. this page describes and. A Catalyst Can Increase The Rate Of A Reaction By.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube A Catalyst Can Increase The Rate Of A Reaction By the rate of a reaction can be increased by adding a suitable catalyst. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. A catalyst does not actually. However, not all reactions have. Catalysts can be homogenous (in the same. this page explains how adding a catalyst affects the rate of. A Catalyst Can Increase The Rate Of A Reaction By.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science A Catalyst Can Increase The Rate Of A Reaction By catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. this change is brought about by a specific interaction between the catalyst and the reaction components. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. It assumes that you are already familiar. A Catalyst Can Increase The Rate Of A Reaction By.
From www.chegg.com
Solved Catalysts increase reaction rates by A) increasing A Catalyst Can Increase The Rate Of A Reaction By catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. this change is brought about by a specific interaction between the catalyst and the reaction components. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. A catalyst is a substance that can. A Catalyst Can Increase The Rate Of A Reaction By.
From learnaboutchemistry.com
Can a Catalyst increase the rate of a reaction? » Learn About Chemistry A Catalyst Can Increase The Rate Of A Reaction By this page describes and explains the way that adding a catalyst affects the rate of a reaction. However, not all reactions have. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. . A Catalyst Can Increase The Rate Of A Reaction By.
From www.chegg.com
Solved A catalyst can increase the rate of a reaction ___. A Catalyst Can Increase The Rate Of A Reaction By this page describes and explains the way that adding a catalyst affects the rate of a reaction. the rate of a reaction can be increased by adding a suitable catalyst. this change is brought about by a specific interaction between the catalyst and the reaction components. Is a substance that increases the rate of reaction, but can. A Catalyst Can Increase The Rate Of A Reaction By.
From learningrovihvu.z19.web.core.windows.net
Graph Of Reaction With And Without Enzyme A Catalyst Can Increase The Rate Of A Reaction By A catalyst does not actually. this page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts can be homogenous (in the same. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance which increases. Is a substance that. A Catalyst Can Increase The Rate Of A Reaction By.
From spmchemistry.blog.onlinetuition.com.my
Factors Affecting the Rate of Reaction SPM Chemistry A Catalyst Can Increase The Rate Of A Reaction By However, not all reactions have. this page describes and explains the way that adding a catalyst affects the rate of a reaction. only a very small mass of catalyst is needed to increase the rate of a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It. A Catalyst Can Increase The Rate Of A Reaction By.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at A Catalyst Can Increase The Rate Of A Reaction By Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. this page explains how adding a catalyst affects the rate of a reaction. the rate of a reaction can be increased by adding a suitable catalyst. It assumes that you are already familiar with basic ideas about the. A catalyst is. A Catalyst Can Increase The Rate Of A Reaction By.
From studyfullfarrior.z19.web.core.windows.net
Rates Of Reaction And Energy Changes A Catalyst Can Increase The Rate Of A Reaction By this change is brought about by a specific interaction between the catalyst and the reaction components. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. Catalysts can be homogenous (in the same. this page explains how adding a catalyst affects the rate of a reaction. only a very small. A Catalyst Can Increase The Rate Of A Reaction By.
From www.sasadoctor.com
Rate of reaction chemistry igcse A Catalyst Can Increase The Rate Of A Reaction By only a very small mass of catalyst is needed to increase the rate of a reaction. this change is brought about by a specific interaction between the catalyst and the reaction components. this page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the.. A Catalyst Can Increase The Rate Of A Reaction By.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint A Catalyst Can Increase The Rate Of A Reaction By only a very small mass of catalyst is needed to increase the rate of a reaction. the rate of a reaction can be increased by adding a suitable catalyst. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. A catalyst is a substance that can help the reactants in a. A Catalyst Can Increase The Rate Of A Reaction By.
From www.gauthmath.com
Solved How does a catalyst increase the rate of a chemical reaction A Catalyst Can Increase The Rate Of A Reaction By A catalyst is a substance which increases. It assumes that you are already familiar with basic ideas about the. this page describes and explains the way that adding a catalyst affects the rate of a reaction. this page explains how adding a catalyst affects the rate of a reaction. Catalysts can be homogenous (in the same. A catalyst. A Catalyst Can Increase The Rate Of A Reaction By.
From www.toppr.com
How do enzymes increase the rate of reaction? A Catalyst Can Increase The Rate Of A Reaction By only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have. this page explains how adding a catalyst affects the rate of a reaction. the rate of a reaction can be increased by adding a suitable catalyst. this change is brought about by a specific interaction. A Catalyst Can Increase The Rate Of A Reaction By.
From www.numerade.com
SOLVEDDoes a catalyst increase reaction rate by the same means as a A Catalyst Can Increase The Rate Of A Reaction By this change is brought about by a specific interaction between the catalyst and the reaction components. only a very small mass of catalyst is needed to increase the rate of a reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst does not actually. Is a substance. A Catalyst Can Increase The Rate Of A Reaction By.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 A Catalyst Can Increase The Rate Of A Reaction By It assumes that you are already familiar with basic ideas about the. only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst does not actually. this page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance which increases. . A Catalyst Can Increase The Rate Of A Reaction By.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free A Catalyst Can Increase The Rate Of A Reaction By catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst does not actually. the rate of a reaction can be increased by adding a suitable catalyst. It assumes that you are already familiar with basic ideas about the. this page explains how adding a catalyst affects the. A Catalyst Can Increase The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Catalyst Can Increase The Rate Of A Reaction By this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Is a substance that. A Catalyst Can Increase The Rate Of A Reaction By.
From www.numerade.com
SOLVEDThe function of catalyst in chemical reaction is to (a) increase A Catalyst Can Increase The Rate Of A Reaction By the rate of a reaction can be increased by adding a suitable catalyst. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance which increases. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It. A Catalyst Can Increase The Rate Of A Reaction By.
From study.com
Effect of Catalysts on Rates of Reaction Lesson A Catalyst Can Increase The Rate Of A Reaction By only a very small mass of catalyst is needed to increase the rate of a reaction. this change is brought about by a specific interaction between the catalyst and the reaction components. this page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts can be homogenous (in the same. It. A Catalyst Can Increase The Rate Of A Reaction By.