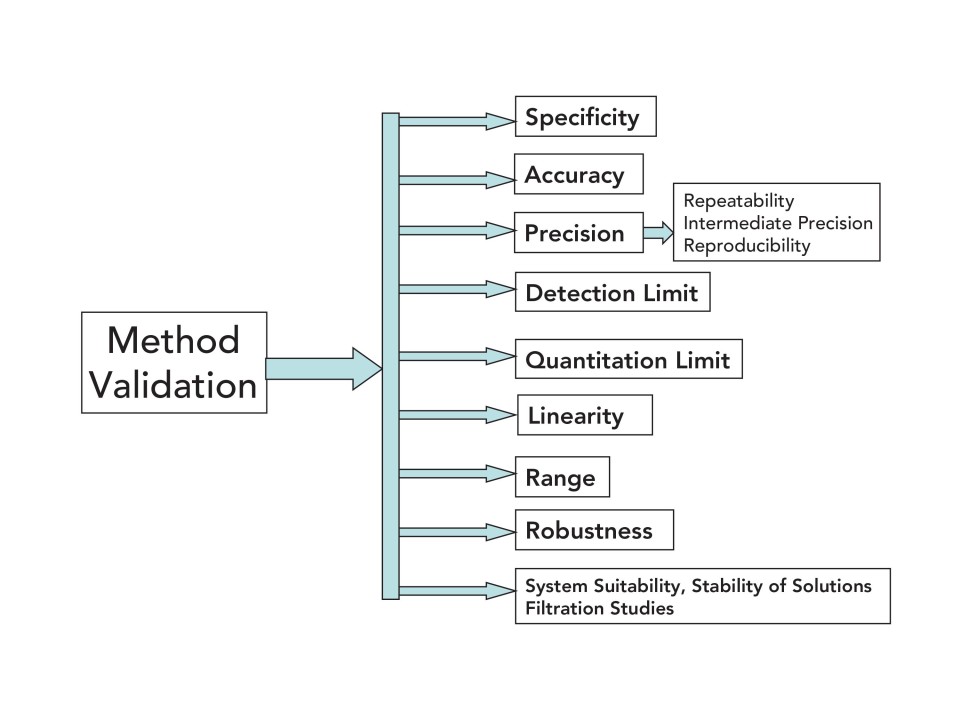
Difference Between Analytical Method Qualification And Validation . method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. 4 analytical procedure is interchangeable with a method or test procedure. Qualification is a part of validation. Ensures the process is capable of producing consistent results within the approved specifications. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 5 compendial methods are verified rather than validated as. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their.
from www.linkedin.com
while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 4 analytical procedure is interchangeable with a method or test procedure. Ensures the process is capable of producing consistent results within the approved specifications. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. 5 compendial methods are verified rather than validated as. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. Qualification is a part of validation.
What Is The Difference Between Verification And Validation Of
Difference Between Analytical Method Qualification And Validation while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. 4 analytical procedure is interchangeable with a method or test procedure. Qualification is a part of validation. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 5 compendial methods are verified rather than validated as. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. Ensures the process is capable of producing consistent results within the approved specifications. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the.
From pharmasiksha.com
Difference between qualification and validation Pharmasiksha Difference Between Analytical Method Qualification And Validation while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. Qualification is a part of validation.. Difference Between Analytical Method Qualification And Validation.
From www.vrogue.co
The Difference Between Qualification And Validation L vrogue.co Difference Between Analytical Method Qualification And Validation while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. Ensures the process is capable of producing consistent results within the approved specifications. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. while qualification and validation share the common goal of ensuring product. Difference Between Analytical Method Qualification And Validation.
From www.youtube.com
Difference Between Qualification and Validation YouTube Difference Between Analytical Method Qualification And Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. Ensures the process is capable of producing consistent results within the approved specifications. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. 5 compendial methods. Difference Between Analytical Method Qualification And Validation.
From pharmaguddu.com
Difference Between Validation, Calibration, and Qualification in Pharma Difference Between Analytical Method Qualification And Validation while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. Qualification is a part of validation. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of. Difference Between Analytical Method Qualification And Validation.
From www.linkedin.com
What Is The Difference Between Verification And Validation Of Difference Between Analytical Method Qualification And Validation method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. 5 compendial methods are verified rather than validated as. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. Ensures the process is capable of producing consistent. Difference Between Analytical Method Qualification And Validation.
From www.vrogue.co
Analytical Method Validation vrogue.co Difference Between Analytical Method Qualification And Validation while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. 5 compendial methods are verified rather than validated as. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. while qualification and validation share the common goal of. Difference Between Analytical Method Qualification And Validation.
From www.slideserve.com
PPT Analytical Methods What, When and How to Validate PowerPoint Difference Between Analytical Method Qualification And Validation while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. 4 analytical procedure is interchangeable with a method or test procedure. Qualification is a part of validation. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. while both. Difference Between Analytical Method Qualification And Validation.
From dheerajpratapsingh0.blogspot.com
Steps for HPLC Method Development Difference Between Analytical Method Qualification And Validation 5 compendial methods are verified rather than validated as. 4 analytical procedure is interchangeable with a method or test procedure. Ensures the process is capable of producing consistent results within the approved specifications. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. while qualification and validation share. Difference Between Analytical Method Qualification And Validation.
From www.a3p.org
Some good validation practices for analytical procedures Difference Between Analytical Method Qualification And Validation 4 analytical procedure is interchangeable with a method or test procedure. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. while ‘validation’ is well understood and recognized. Difference Between Analytical Method Qualification And Validation.
From pharmagxp.com
9 Important Differences Between Qualification and Validation Pharma GxP Difference Between Analytical Method Qualification And Validation 4 analytical procedure is interchangeable with a method or test procedure. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. 5 compendial methods are verified rather than validated as. while qualification and validation share the common goal of ensuring product quality and regulatory compliance,. Difference Between Analytical Method Qualification And Validation.
From www.vrogue.co
The Difference Between Qualification And Validation V vrogue.co Difference Between Analytical Method Qualification And Validation qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 5 compendial methods are verified rather than validated as. 4 analytical procedure is interchangeable with a method or test procedure. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. method validation is the. Difference Between Analytical Method Qualification And Validation.
From www.vrogue.co
The Difference Between Qualification And Validation V vrogue.co Difference Between Analytical Method Qualification And Validation while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. Qualification is a part of validation. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the.. Difference Between Analytical Method Qualification And Validation.
From sanninodario.altervista.org
"Validation" Or "Qualification" What's The Difference? Pharma Difference Between Analytical Method Qualification And Validation while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 4 analytical procedure is interchangeable with a method or test procedure. while ‘validation’ is well understood and recognized across the industry, the other terms. Difference Between Analytical Method Qualification And Validation.
From gmpinsiders.com
Qualification Vs Validation Understand The Key Differences Difference Between Analytical Method Qualification And Validation Ensures the process is capable of producing consistent results within the approved specifications. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. while ‘validation’ is well. Difference Between Analytical Method Qualification And Validation.
From www.semanticscholar.org
Figure 2 from Review Article Overview of Validation and Basic Concepts Difference Between Analytical Method Qualification And Validation while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. Qualification is a part of validation. Ensures the process is capable of producing consistent results within the approved specifications. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their.. Difference Between Analytical Method Qualification And Validation.
From www.learngxp.com
The Difference Between Qualification and Validation LearnGxP Difference Between Analytical Method Qualification And Validation 5 compendial methods are verified rather than validated as. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. Ensures the process is capable of producing consistent results within. Difference Between Analytical Method Qualification And Validation.
From www.m-pharmaguide.com
Analytical Method Validation Protocol for Nystatin Difference Between Analytical Method Qualification And Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. 5 compendial methods are verified rather than validated as. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 4 analytical procedure is interchangeable with a method or test procedure. Qualification is a. Difference Between Analytical Method Qualification And Validation.
From www.slideserve.com
PPT ANALYTICAL METHOD VALIDATION PowerPoint Presentation, free Difference Between Analytical Method Qualification And Validation method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. Qualification is. Difference Between Analytical Method Qualification And Validation.
From gmpinsiders.com
Analytical Method Validation How It Affects Testing Integrity And Why Difference Between Analytical Method Qualification And Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. 4 analytical procedure is interchangeable with a method or test procedure. qualification and validation. Difference Between Analytical Method Qualification And Validation.
From www.americanpharmaceuticalreview.com
Analytical Method Validation for Quality Assurance and Process Difference Between Analytical Method Qualification And Validation while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. Qualification is a part of validation. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in. Difference Between Analytical Method Qualification And Validation.
From www.slideserve.com
PPT Analytical Method Validation PowerPoint Presentation, free Difference Between Analytical Method Qualification And Validation while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. 4 analytical procedure is interchangeable with a method or test procedure. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. Ensures the process is capable of producing consistent results. Difference Between Analytical Method Qualification And Validation.
From www.chemwifi.com
Analytical Method validation for Pesticides, PCBs, PAHs Phthalates Difference Between Analytical Method Qualification And Validation while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. 4 analytical procedure is interchangeable. Difference Between Analytical Method Qualification And Validation.
From www.youtube.com
Difference Between Qualification and Validation Qualification Vs Difference Between Analytical Method Qualification And Validation method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. Ensures the process is capable of producing consistent results within the approved specifications. 4 analytical procedure is interchangeable with a method or test procedure. 5 compendial methods are verified rather than validated as. while ‘validation’. Difference Between Analytical Method Qualification And Validation.
From www.slideserve.com
PPT Analytical Methods What, When and How to Validate PowerPoint Difference Between Analytical Method Qualification And Validation Qualification is a part of validation. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 5 compendial methods are verified rather than validated as. method validation is the process by which it. Difference Between Analytical Method Qualification And Validation.
From www.slideserve.com
PPT Analytical Method Validation PowerPoint Presentation, free Difference Between Analytical Method Qualification And Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 5 compendial methods are verified rather than validated as. while ‘validation’ is well understood and recognized across the industry, the other terms are. Difference Between Analytical Method Qualification And Validation.
From gxpcellators.com
Validation vs Qualification in Pharmaceutical Industry Difference Between Analytical Method Qualification And Validation Qualification is a part of validation. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. Ensures the process is capable of producing consistent results within. Difference Between Analytical Method Qualification And Validation.
From www.slideshare.net
Analytical method validation Difference Between Analytical Method Qualification And Validation while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. Qualification is a part of validation. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the.. Difference Between Analytical Method Qualification And Validation.
From www.slideshare.net
analytical method validation and validation of hplc Difference Between Analytical Method Qualification And Validation while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. 5 compendial methods are verified rather than validated as. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 4 analytical procedure is interchangeable with a method or test procedure. while ‘validation’ is well. Difference Between Analytical Method Qualification And Validation.
From www.youtube.com
VALIDATION OF ANALYTICAL METHOD Method validation Validation of an Difference Between Analytical Method Qualification And Validation 5 compendial methods are verified rather than validated as. Ensures the process is capable of producing consistent results within the approved specifications. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. while ‘validation’ is well understood and recognized across the industry, the other terms are. Difference Between Analytical Method Qualification And Validation.
From www.linkedin.com
9 Most Important Parameter for Analytical method validation Difference Between Analytical Method Qualification And Validation Qualification is a part of validation. 4 analytical procedure is interchangeable with a method or test procedure. while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. while both. Difference Between Analytical Method Qualification And Validation.
From www.slideshare.net
Analytical method validation Difference Between Analytical Method Qualification And Validation 5 compendial methods are verified rather than validated as. Ensures the process is capable of producing consistent results within the approved specifications. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. while. Difference Between Analytical Method Qualification And Validation.
From pharmagxp.com
9 Important Differences Between Qualification and Validation Pharma GxP Difference Between Analytical Method Qualification And Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. Ensures the process is capable of producing consistent results within the approved specifications. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. 5 compendial methods are verified rather than. Difference Between Analytical Method Qualification And Validation.
From gmpinsiders.com
Qualification Vs Validation Understand The Key Differences Difference Between Analytical Method Qualification And Validation while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. Qualification is a part of validation. Ensures the process is capable of producing consistent results within the approved specifications. 4 analytical procedure is interchangeable with a method or test procedure. while ‘validation’ is well understood and recognized across the. Difference Between Analytical Method Qualification And Validation.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Difference Between Analytical Method Qualification And Validation Ensures the process is capable of producing consistent results within the approved specifications. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. Qualification is a part of validation. while qualification and validation share the common goal of ensuring product quality and regulatory compliance, there are key. method validation is the process by. Difference Between Analytical Method Qualification And Validation.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Difference Between Analytical Method Qualification And Validation while ‘validation’ is well understood and recognized across the industry, the other terms are sometimes used in wrong context. qualification and validation studies are essential processes in the pharmaceutical industry that ensure the. 5 compendial methods are verified rather than validated as. Qualification is a part of validation. Ensures the process is capable of producing consistent results within. Difference Between Analytical Method Qualification And Validation.