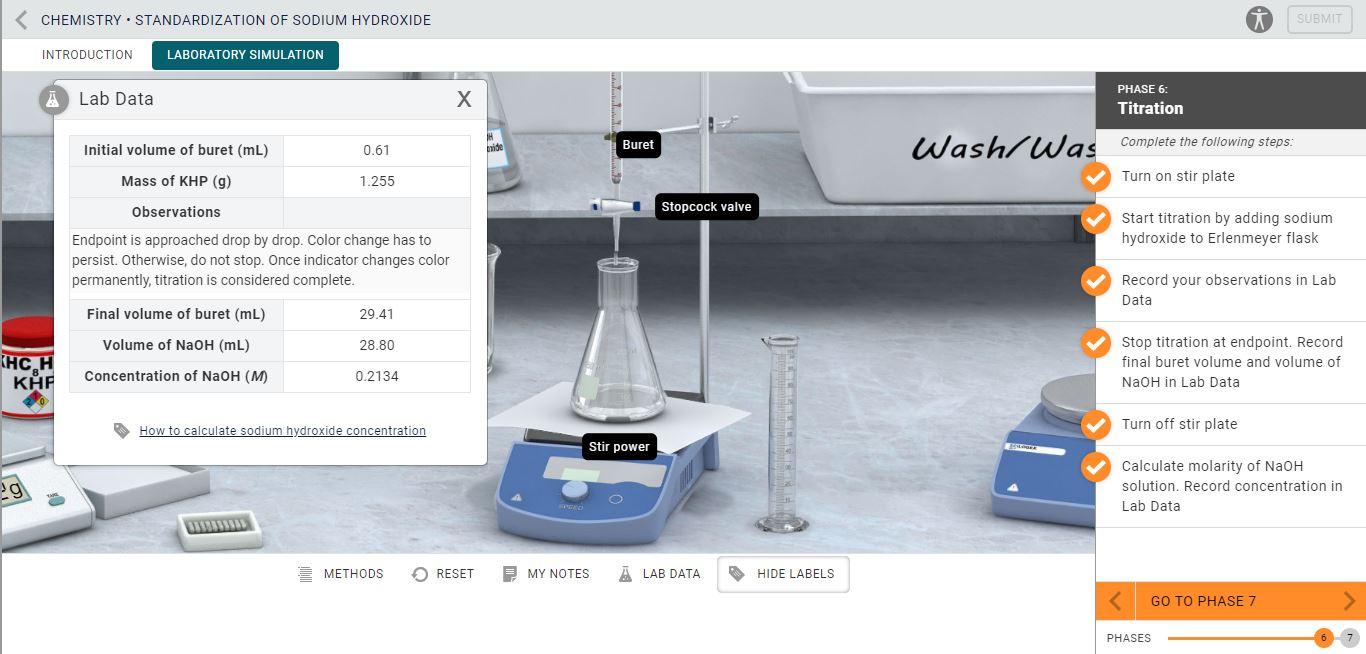
Titration Lab Table . a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Table \(\pageindex{1}\) shows the four types of titrations, and you note. You will also learn about equivalence points and endpoints, and titration calculations. The reagent (titrant) is the solution with a known molarity that will react with the analyte.
from www.chegg.com
You will also learn about equivalence points and endpoints, and titration calculations. Table \(\pageindex{1}\) shows the four types of titrations, and you note. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. The reagent (titrant) is the solution with a known molarity that will react with the analyte. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.
TitrationStandardization of Sodium Hydroxide
Titration Lab Table The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown molarity. Table \(\pageindex{1}\) shows the four types of titrations, and you note. You will also learn about equivalence points and endpoints, and titration calculations. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.
From mungfali.com
Titration Lab Diagram Titration Lab Table The reagent (titrant) is the solution with a known molarity that will react with the analyte. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Table \(\pageindex{1}\) shows the four types of titrations, and you note. a titration is an analytical procedure used to determine the accurate concentration of. Titration Lab Table.
From www.scribd.com
LAB Report 3 Titration Chemistry Titration Lab Table in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. You. Titration Lab Table.
From www.chegg.com
Solved Summary Data Table AcidBase Titration CHEZ 101L Titration Lab Table in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with. Titration Lab Table.
From www.rdm-ind.com
RDM Laboratory Table with Suspended A109PLABCAB (Flat Top Titration Lab Table a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. You will also learn about equivalence points and endpoints, and titration calculations.. Titration Lab Table.
From studylib.net
CHEMISTRY I LAB INTRO TO TITRATION Titration Lab Table You will also learn about equivalence points and endpoints, and titration calculations. The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is an analytical procedure used to determine the accurate concentration of a sample. Titration Lab Table.
From modernalternativemama.com
lab report titration Titration Lab Table The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown molarity. titration is the experimental method of determining a solution of unknown. Titration Lab Table.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Lab Table The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this tutorial, you will. Titration Lab Table.
From www.studocu.com
Titration Lab Report Acid Base Titration Lab Report Table 3 NaOH Titration Lab Table titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. Table \(\pageindex{1}\) shows the four types of titrations, and you note. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown. Titration Lab Table.
From dxojrsulq.blob.core.windows.net
How To Report A Titration Experiment at Joseph Heitmann blog Titration Lab Table in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. The analyte (titrand) is the solution with an unknown molarity. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. You will also learn about equivalence points and endpoints, and titration calculations. The. Titration Lab Table.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Titration Lab Table titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Table \(\pageindex{1}\) shows the four types of titrations, and you. Titration Lab Table.
From dxowzxmzk.blob.core.windows.net
Titration Table Example at Richard Sherman blog Titration Lab Table The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. You will also learn about equivalence points and endpoints, and titration calculations. Table \(\pageindex{1}\) shows the four types of titrations, and you. Titration Lab Table.
From www.numerade.com
SOLVED 5 Use table to record your titration data in the data and Titration Lab Table titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. You. Titration Lab Table.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Lab Table titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. Table \(\pageindex{1}\) shows the four types of titrations, and you note. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this tutorial, you will learn about titration. Titration Lab Table.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Lab Table You will also learn about equivalence points and endpoints, and titration calculations. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown molarity. Table \(\pageindex{1}\) shows the four types of titrations, and you note. in this tutorial, you. Titration Lab Table.
From www.chegg.com
Solved Section Titration Lab Report Sheet Table 1 Acid Base Titration Lab Table in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Table \(\pageindex{1}\) shows the four types of titrations, and you note. titration is the experimental method of determining. Titration Lab Table.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Lab Table Table \(\pageindex{1}\) shows the four types of titrations, and you note. You will also learn about equivalence points and endpoints, and titration calculations. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. The analyte (titrand) is the solution with an unknown molarity. in this tutorial, you will learn about titration. Titration Lab Table.
From studylib.net
Titration Lab Report Walker Singleton Titration Lab Table Table \(\pageindex{1}\) shows the four types of titrations, and you note. The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The reagent (titrant) is the solution with a known molarity that will react with the analyte. . Titration Lab Table.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Titration Lab Table a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this tutorial, you will learn about titration curves, titration analysis and the steps required. Titration Lab Table.
From www.vrogue.co
Titration Data Sheetmethod 1 Titration With Buret An vrogue.co Titration Lab Table The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is an analytical procedure used to determine the accurate. Titration Lab Table.
From exoqbgfse.blob.core.windows.net
Sample Lab Report On Acid Base Titration at Michelle Hamilton blog Titration Lab Table The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. a titration is an analytical procedure used to determine. Titration Lab Table.
From brainly.com
Titration Lab Sheet Day 2 (ALTERNATE) Titration Lab Table Table \(\pageindex{1}\) shows the four types of titrations, and you note. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by. Titration Lab Table.
From dxowzxmzk.blob.core.windows.net
Titration Table Example at Richard Sherman blog Titration Lab Table in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure. Titration Lab Table.
From www.studocu.com
Lab Technique 3 AcidBase Titration Post Lab Lab Technique 3 Acid Titration Lab Table You will also learn about equivalence points and endpoints, and titration calculations. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this tutorial,. Titration Lab Table.
From mstimms-btecmedicalscience12.blogspot.com
BTEC Medical science titration results table Titration Lab Table You will also learn about equivalence points and endpoints, and titration calculations. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown molarity. Table \(\pageindex{1}\) shows the four types of titrations, and you note. titration is the experimental. Titration Lab Table.
From www.labtechsupplyco.com
Custom Lab Table Science Lab Furniture LabTech Supply Titration Lab Table in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Table \(\pageindex{1}\) shows the four types of titrations, and you note. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The reagent (titrant) is the solution with a. Titration Lab Table.
From mavink.com
Titration Lab Setup Titration Lab Table Table \(\pageindex{1}\) shows the four types of titrations, and you note. The reagent (titrant) is the solution with a known molarity that will react with the analyte. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. in this tutorial, you will learn about titration curves, titration analysis and the steps. Titration Lab Table.
From www.chegg.com
Solved Titration post lab complete the table using the Titration Lab Table titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. The analyte (titrand) is the solution with an unknown molarity. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about equivalence points and endpoints, and titration calculations. Table. Titration Lab Table.
From www.numerade.com
SOLVED The following data is from a conductometric titration of 10.00 Titration Lab Table a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown molarity. Table \(\pageindex{1}\) shows the four types of titrations, and you note. in this tutorial, you will learn about titration curves, titration analysis and the steps required to. Titration Lab Table.
From dxompsokn.blob.core.windows.net
Lab Manual Titration at Charles Fisher blog Titration Lab Table a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with. Titration Lab Table.
From mavink.com
Clozapine Titration Chart Titration Lab Table a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Table \(\pageindex{1}\) shows the four types of titrations, and you note. You will also learn about equivalence points and endpoints, and titration. Titration Lab Table.
From www.chegg.com
Solved Chem 1 Lab Redox Titration and Determination of Fe. Titration Lab Table in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. You will also learn about equivalence points and endpoints, and titration calculations. The analyte (titrand) is the solution with. Titration Lab Table.
From rebeccasouthtitrationlab.weebly.com
Pictures and Video Titration Lab Titration Lab Table The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. a titration is. Titration Lab Table.
From www.chegg.com
This is a titration table and we have 50.0ml 0.1319M Titration Lab Table titration is the experimental method of determining a solution of unknown concentration by the controlled addition of. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown molarity. Table \(\pageindex{1}\) shows the four types of titrations, and you. Titration Lab Table.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Lab Table The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Table \(\pageindex{1}\) shows the four types of titrations, and you note. a titration is an analytical procedure used to determine the accurate concentration of a sample by. Titration Lab Table.
From www.chegg.com
TitrationStandardization of Sodium Hydroxide Titration Lab Table a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about equivalence points and endpoints, and titration calculations. a titration is an analytical procedure. Titration Lab Table.