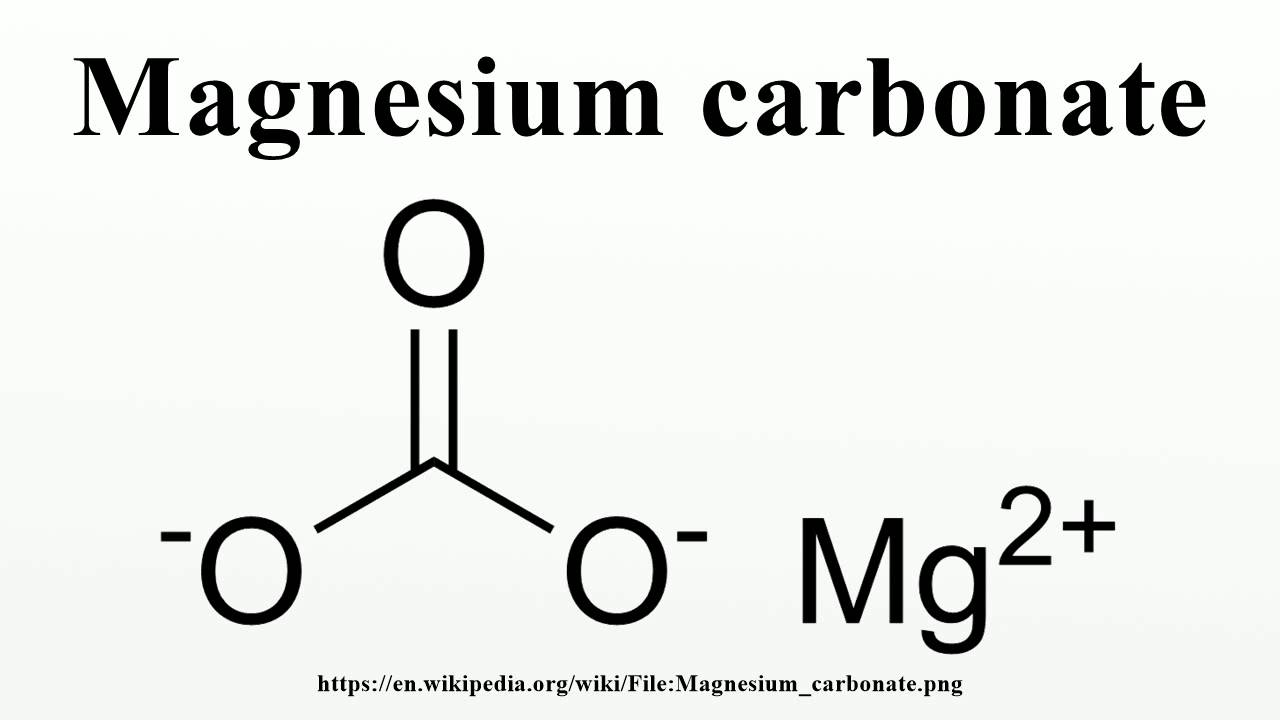Magnesium Carbonate When Heated . When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. It explains how the thermal. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. When magnesium carbonate is heated, it dissociates into mgo and co₂. There is no change in colour because. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide.
from www.youtube.com
Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. There is no change in colour because. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. When magnesium carbonate is heated, it dissociates into mgo and co₂. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. It explains how the thermal. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon.
Magnesium carbonate YouTube
Magnesium Carbonate When Heated When magnesium carbonate is heated, it dissociates into mgo and co₂. When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. There is no change in colour because. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. It explains how the thermal. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. When magnesium carbonate is heated, it dissociates into mgo and co₂.
From byjus.com
20.0 g of a magnesium carbonate sample on heating to give Magnesium Carbonate When Heated When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. It explains how the thermal. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When magnesium carbonate is heated, it dissociates into mgo and co₂. When magnesium. Magnesium Carbonate When Heated.
From www.degruyter.com
Synthesis of magnesium carbonate hydrate from natural talc Magnesium Carbonate When Heated This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a. Magnesium Carbonate When Heated.
From www.shutterstock.com
23 Magnesium Carbonate Formula Images, Stock Photos & Vectors Magnesium Carbonate When Heated There is no change in colour because. When magnesium carbonate is heated, it dissociates into mgo and co₂. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. It explains how the thermal. When heated beyond its decomposition temperature,. Magnesium Carbonate When Heated.
From www.savemyexams.co.uk
Carbon Dioxide from Thermal (2.3.4) Edexcel IGCSE Magnesium Carbonate When Heated There is no change in colour because. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. It explains how the thermal. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. This page examines at the effect of heat on the carbonates and nitrates of the group 2. Magnesium Carbonate When Heated.
From setarvan.com
MAGNESIUM CARBONATE Magnesium Carbonate When Heated There is no change in colour because. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. When magnesium carbonate is heated, it dissociates into mgo and co₂. Mgco₃ → mgo. Magnesium Carbonate When Heated.
From www.numerade.com
The Group IIA carbonates when heated. For example, MgCO3(s Magnesium Carbonate When Heated This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. It explains how the thermal. When heated. Magnesium Carbonate When Heated.
From www.dreamstime.com
Magnesium Carbonate Molecule Stock Vector Illustration of Magnesium Carbonate When Heated When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. When magnesium carbonate is heated, it dissociates into mgo and co₂. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. There is no change in colour because. This page examines at the effect of heat on. Magnesium Carbonate When Heated.
From www.slideshare.net
Action of heat on chemical compound e book Magnesium Carbonate When Heated This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). There is no change in colour because. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. When magnesium carbonate is heated, it dissociates into mgo and co₂.. Magnesium Carbonate When Heated.
From pediaa.com
What is the Difference Between Light and Heavy Magnesium Carbonate Magnesium Carbonate When Heated Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. There is no change in colour because. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. When magnesium carbonate is heated, it dissociates into mgo and co₂.. Magnesium Carbonate When Heated.
From www.toppr.com
20.0 mg of a magnesium carbonate sample on heating to give Magnesium Carbonate When Heated It explains how the thermal. When magnesium carbonate is heated, it dissociates into mgo and co₂. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. There is no change in colour because. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless. Magnesium Carbonate When Heated.
From pubs.acs.org
HeatInduced Dolomitization of Amorphous Calcium Magnesium Carbonate in Magnesium Carbonate When Heated When magnesium carbonate is heated, it dissociates into mgo and co₂. There is no change in colour because. When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. When magnesium carbonate is heated, it breaks down to produce magnesium. Magnesium Carbonate When Heated.
From glchems.en.made-in-china.com
High Purity Chemical Light Magnesium Carbonate Industrial Grade Magnesium Carbonate When Heated When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. When magnesium carbonate is heated, it dissociates into mgo and co₂. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. There is. Magnesium Carbonate When Heated.
From www.alamy.com
Rock of magnesium carbonate Stock Photo Alamy Magnesium Carbonate When Heated When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. This page examines at the effect of heat on the carbonates. Magnesium Carbonate When Heated.
From retail.vitalbulk.com
Magnesium Carbonate 29 Powder VitalBulk Inc. Retail Magnesium Carbonate When Heated When magnesium carbonate is heated, it dissociates into mgo and co₂. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. It explains how the thermal. There is no change in colour because. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless. Magnesium Carbonate When Heated.
From www.researchgate.net
(PDF) Hydration of Magnesium Carbonate in a Thermal Energy Storage Magnesium Carbonate When Heated This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. When mgco3 (magnesium carbonate) is. Magnesium Carbonate When Heated.
From www.slideshare.net
Action of heat on chemical compound e book Magnesium Carbonate When Heated It explains how the thermal. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. There is no change in colour because. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. Magnesium and. Magnesium Carbonate When Heated.
From www.youtube.com
Magnesium carbonate light/heavy Labh Group YouTube Magnesium Carbonate When Heated When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. When magnesium carbonate is heated, it dissociates into mgo and co₂. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. This page examines at the. Magnesium Carbonate When Heated.
From brainly.in
When 8.4 g of magnesium carbonate is heated, into Magnesium Carbonate When Heated This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When magnesium carbonate is heated, it dissociates into mgo and co₂. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. When heated beyond its decomposition temperature, magnesium carbonate undergoes a. Magnesium Carbonate When Heated.
From www.slideshare.net
Action of heat on chemical compound Magnesium Carbonate When Heated There is no change in colour because. When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. It explains how the thermal. Magnesium and calcium nitrates normally have water of crystallisation, and the solid. Magnesium Carbonate When Heated.
From sciencekitstore.com
Magnesium Carbonate, Basic, USP, "Extra Light" (32 fl oz or 950 cc Magnesium Carbonate When Heated When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When magnesium carbonate is heated,. Magnesium Carbonate When Heated.
From brainly.in
balanced chemical equation of magnesium reacts with oxygen &calcium Magnesium Carbonate When Heated There is no change in colour because. It explains how the thermal. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. Magnesium and. Magnesium Carbonate When Heated.
From www.healthydirections.com
Magnesium Carbonate What is it and How Does it Effect The Body Magnesium Carbonate When Heated There is no change in colour because. It explains how the thermal. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in. Magnesium Carbonate When Heated.
From www.tradekorea.com
Magnesium Carbonate tradekorea Magnesium Carbonate When Heated It explains how the thermal. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. When magnesium carbonate is heated, it dissociates into mgo and co₂. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. This page examines at the effect of heat on the carbonates and nitrates of the group. Magnesium Carbonate When Heated.
From naturalcalm.ca
Magnesium Carbonate Your Guide to This Type of Magnesium Supplement Magnesium Carbonate When Heated When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. When magnesium carbonate is heated, it dissociates into mgo and co₂. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. There is no change in colour because. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium. Magnesium Carbonate When Heated.
From www.cphi-online.com
Pharmaceutical Grade heavy magnesium Carbonate Hebei Meishen Magnesium Carbonate When Heated Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. It explains how the thermal. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it. Magnesium Carbonate When Heated.
From www.hbarsci.com
Light Magnesium Carbonate, 100g The Curated Chemical Collection — hBARSCI Magnesium Carbonate When Heated Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When magnesium carbonate is heated, it breaks. Magnesium Carbonate When Heated.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Magnesium Carbonate When Heated When magnesium carbonate is heated, it dissociates into mgo and co₂. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. There is no change in colour because. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). Mgco₃ → mgo. Magnesium Carbonate When Heated.
From www.youtube.com
Magnesium carbonate YouTube Magnesium Carbonate When Heated When magnesium carbonate is heated, it dissociates into mgo and co₂. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. This page. Magnesium Carbonate When Heated.
From joildreqq.blob.core.windows.net
Magnesium Bicarbonate + Hydrochloric Acid at Gregory Sanders blog Magnesium Carbonate When Heated When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. There is no change in colour because. It explains how the thermal. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). Magnesium and calcium nitrates normally have water of crystallisation,. Magnesium Carbonate When Heated.
From www.numerade.com
SOLVED Suggest why a pure sample of magnesium carbonate will not be Magnesium Carbonate When Heated When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When magnesium carbonate is heated, it dissociates into mgo and co₂. Magnesium and calcium nitrates normally have water. Magnesium Carbonate When Heated.
From bmp-brah.blogspot.com
Mgco3 Balanced Equation bmpbrah Magnesium Carbonate When Heated When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. There is no change in colour because. When magnesium carbonate is heated, it dissociates into mgo and co₂. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium. Magnesium Carbonate When Heated.
From www.researchgate.net
Chemical composition of the magnesium carbonate (MgCO 3 ), magnesium Magnesium Carbonate When Heated Magnesium and calcium nitrates normally have water of crystallisation, and the solid may dissolve in its own water of crystallisation to make a colourless solution before it starts to. It explains how the thermal. There is no change in colour because. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium,. Magnesium Carbonate When Heated.
From projectopenletter.com
What Happens When Magnesium Ribbon Burns In Air Identify The Type Of Magnesium Carbonate When Heated When heated beyond its decomposition temperature, magnesium carbonate undergoes a chemical reaction to form magnesium oxide and carbon. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. There is no change in colour because. It explains how the thermal. When magnesium carbonate is heated, it dissociates into mgo and co₂. When. Magnesium Carbonate When Heated.
From www.slideshare.net
Action of heat on chemical compound e book Magnesium Carbonate When Heated When magnesium carbonate is heated, it dissociates into mgo and co₂. This page examines at the effect of heat on the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium). When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. There is no change in colour because.. Magnesium Carbonate When Heated.
From www.youtube.com
20 g of magnesium carbonate sample on heating to give carbon Magnesium Carbonate When Heated Mgco₃ → mgo + co₂ again, similar to other group 2 metals, magnesium. When magnesium carbonate is heated, it dissociates into mgo and co₂. When mgco3 (magnesium carbonate) is heated, it will decompose into magnesium oxide (mgo) and carbon dioxide (co2) gas. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. This page examines. Magnesium Carbonate When Heated.