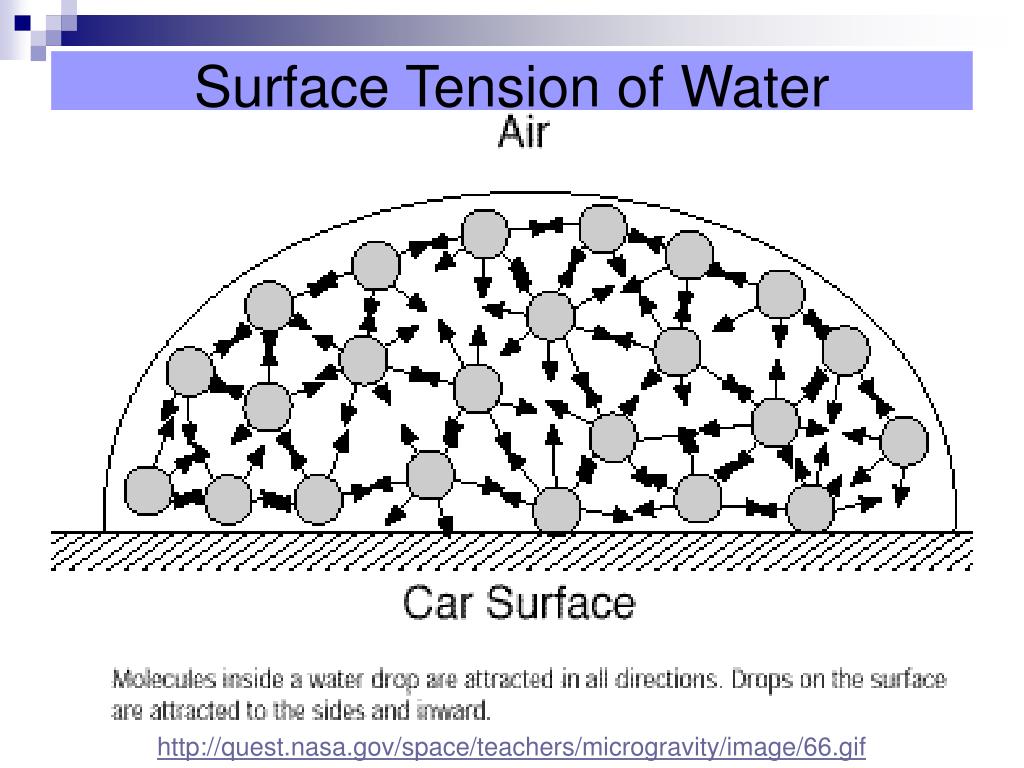
What Happens During Surface Tension . The molecules on the surface are. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This term is typically used only. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force.
from www.slideserve.com
Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The molecules on the surface are. Since these intermolecular forces vary depending on the nature of the. This term is typically used only. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
PPT Water and Aqueous Systems PowerPoint Presentation, free download
What Happens During Surface Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The molecules on the surface are. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This term is typically used only. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid.
From www.pinterest.com
As Condensation Droplets Form and Combine, What Forces are at Work What Happens During Surface Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension is caused by liquid. What Happens During Surface Tension.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid What Happens During Surface Tension Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is the energy, or work, required to increase the surface area of. What Happens During Surface Tension.
From www.learnatnoon.com
What is Surface Tension in Physics? What Happens During Surface Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Since these intermolecular forces vary depending on the nature of the. This. What Happens During Surface Tension.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What Happens During Surface Tension Since these intermolecular forces vary depending on the nature of the. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. What Happens During Surface Tension.
From www.linkedin.com
What is Surface Tension? What Happens During Surface Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is caused by liquid particle. What Happens During Surface Tension.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it What Happens During Surface Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of. What Happens During Surface Tension.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free What Happens During Surface Tension Since these intermolecular forces vary depending on the nature of the. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The. What Happens During Surface Tension.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector What Happens During Surface Tension “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Surface tension is the energy, or work, required to increase the surface area of a. What Happens During Surface Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Happens During Surface Tension Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension is a phenomenon in which the surface of a. What Happens During Surface Tension.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint What Happens During Surface Tension Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a. What Happens During Surface Tension.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit What Happens During Surface Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of the surface film of a liquid caused. What Happens During Surface Tension.
From www.youtube.com
Surface Tension of Water Explained YouTube What Happens During Surface Tension This term is typically used only. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface are. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the. What Happens During Surface Tension.
From www.mdpi.com
Materials Free FullText Predictive Model for the Surface Tension What Happens During Surface Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, property of a liquid surface displayed by its acting as if. What Happens During Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Happens During Surface Tension “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This term is typically used only. Surface tension is a phenomenon in. What Happens During Surface Tension.
From www.studypool.com
SOLUTION Surface tension of milk Studypool What Happens During Surface Tension “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. The surface tension of a liquid is mainly a force that mainly acts to reduce. What Happens During Surface Tension.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL What Happens During Surface Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The molecules on the surface are. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is caused by liquid particle. What Happens During Surface Tension.
From www.engineeringinhindi.com
Surface tension in Hindi What Happens During Surface Tension “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension is caused by liquid particle intermolecular forces such. What Happens During Surface Tension.
From proper-cooking.info
Surface Tension Examples In Nature What Happens During Surface Tension The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. What Happens During Surface Tension.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids What Happens During Surface Tension Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Since these intermolecular forces vary depending on the nature of the. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension is a phenomenon in which the surface of a liquid,. What Happens During Surface Tension.
From delprofedefisica.blogspot.com
Física Tensión superficial What Happens During Surface Tension “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Surface tension, property of a liquid surface displayed by its acting as if it were. What Happens During Surface Tension.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments What Happens During Surface Tension Since these intermolecular forces vary depending on the nature of the. This term is typically used only. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is the energy, or work, required to increase the surface area of a liquid. What Happens During Surface Tension.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation, free download What Happens During Surface Tension The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. The molecules on the surface are. Surface tension is a. What Happens During Surface Tension.
From www.worksheetsplanet.com
What is Surface Tension? What Happens During Surface Tension Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This term is typically used only. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as. What Happens During Surface Tension.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Happens During Surface Tension The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. What Happens During Surface Tension.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint What Happens During Surface Tension This term is typically used only. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. The molecules on the surface are. “surface tension is the tension of the surface film of a. What Happens During Surface Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Happens During Surface Tension “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. The molecules on the. What Happens During Surface Tension.
From www.dreamstime.com
Surface Tension stock vector. Illustration of environment 92206295 What Happens During Surface Tension Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Since these intermolecular forces vary depending on the nature of the. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface. What Happens During Surface Tension.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation What Happens During Surface Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Since these intermolecular forces vary depending on the nature of the. “surface tension is the tension of. What Happens During Surface Tension.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse What Happens During Surface Tension Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface are. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Surface tension, property of a liquid surface. What Happens During Surface Tension.
From bio1100.nicerweb.com
surface_tension/02_13 What Happens During Surface Tension Since these intermolecular forces vary depending on the nature of the. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. The molecules on the surface are.. What Happens During Surface Tension.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and What Happens During Surface Tension “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid,. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the.. What Happens During Surface Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Happens During Surface Tension Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid.. What Happens During Surface Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Happens During Surface Tension The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. This term is typically used only. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of the surface film of a liquid caused. What Happens During Surface Tension.
From www.youtube.com
Surface Tension YouTube What Happens During Surface Tension Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This term is typically used only. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Surface tension, property of a liquid surface displayed by its acting as if. What Happens During Surface Tension.
From byjus.com
Explain the surface tension phenomenon with examples. What Happens During Surface Tension Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in. What Happens During Surface Tension.