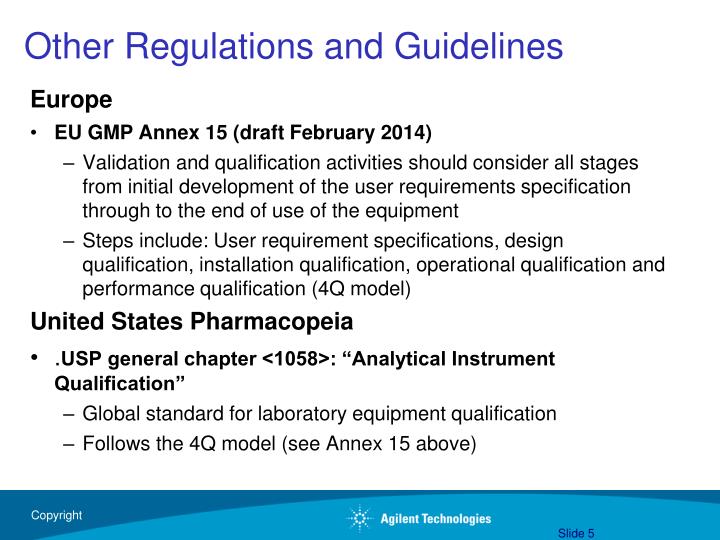
Instrument Qualification Guidelines . Design qualification (dq), installation qualification dure will generate test. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. The iq outlines procedures used to confirm the. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. An installation qualification (iq) and an operational qualification (oq).
from www.slideserve.com
Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. An installation qualification (iq) and an operational qualification (oq). Design qualification (dq), installation qualification dure will generate test. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. The iq outlines procedures used to confirm the. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an.
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters
Instrument Qualification Guidelines Design qualification (dq), installation qualification dure will generate test. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Design qualification (dq), installation qualification dure will generate test. An installation qualification (iq) and an operational qualification (oq). Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. The iq outlines procedures used to confirm the. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your.
From cewuupel.blob.core.windows.net
Equipment Qualification Guidelines Pics at Floyd Jeffries blog Instrument Qualification Guidelines I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. The iq outlines procedures used to confirm the. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification. Instrument Qualification Guidelines.
From www.researchgate.net
(PDF) Qualification Guidelines for Personal ComputerBased Aviation Training Devices Instrument Instrument Qualification Guidelines Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Design qualification (dq), installation qualification dure will generate test. An installation qualification (iq) and an operational qualification (oq). I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. An installation qualification (iq) and an operational qualification (oq). Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. The iq outlines procedures used to confirm the. Design qualification (dq), installation qualification dure. Instrument Qualification Guidelines.
From www.presentationeze.com
Equipment Validation Facility Qualification Material QualificationPresentationEZE Instrument Qualification Guidelines I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. An installation qualification (iq) and an operational qualification (oq). The iq outlines procedures used to confirm the. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv). Instrument Qualification Guidelines.
From pharmablog.in
Qualification of Equipment / Instrument / Utilities SOP PharmaBlog Instrument Qualification Guidelines An installation qualification (iq) and an operational qualification (oq). Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Installation qualification (iq), operational qualification (oq),. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Design qualification (dq), installation qualification dure will generate test. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. The iq outlines procedures used to confirm the. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. Analytical instrument qualification. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines An installation qualification (iq) and an operational qualification (oq). Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. The iq outlines procedures used to confirm the. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Design qualification (dq), installation qualification dure will generate. Instrument Qualification Guidelines.
From www.slideshare.net
Analytical Instrument Qualification and System Validation Instrument Qualification Guidelines The iq outlines procedures used to confirm the. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. The iq outlines procedures used to confirm the. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Design qualification (dq), installation qualification dure will generate test. An installation qualification (iq) and an operational qualification. Instrument Qualification Guidelines.
From www.semanticscholar.org
AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF PHARMACEUTICAL INDUSTRY Instrument Qualification Guidelines An installation qualification (iq) and an operational qualification (oq). Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. The iq outlines procedures used to confirm the. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. Us pharmacopeia (usp) general chapter. Instrument Qualification Guidelines.
From www.slideserve.com
PPT Quality Assurance and Regulatory Compliance for Pharmaceutical Product PowerPoint Instrument Qualification Guidelines The iq outlines procedures used to confirm the. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. Us pharmacopeia (usp) general. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. Analytical instrument qualification aiq is the collection of documented evidence that an. Instrument Qualification Guidelines.
From www.spectroscopyeurope.com
Instrument qualification a possible quality by designbased approach Spectroscopy Europe/World Instrument Qualification Guidelines Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Design qualification (dq), installation qualification dure will generate test. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. An installation qualification (iq) and an operational qualification (oq). I have reviewed key parts of usp on analytical instrument qualification to highlight areas. Instrument Qualification Guidelines.
From www.getreskilled.com
Equipment Qualification Protocol Step by Step Writing Guide Instrument Qualification Guidelines I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. An installation qualification (iq) and an operational qualification (oq). Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Installation qualification (iq) is the. Instrument Qualification Guidelines.
From www.cytivalifesciences.com
Stay compliant instrument qualification for cGMP Cytiva Instrument Qualification Guidelines I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. An installation qualification (iq) and an operational qualification (oq). Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Installation qualification (iq), operational qualification. Instrument Qualification Guidelines.
From www.drugfuture.com
ANALYTICAL INSTRUMENT QUALIFICATION Instrument Qualification Guidelines Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Us pharmacopeia. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines An installation qualification (iq) and an operational qualification (oq). The iq outlines procedures used to confirm the. Design qualification (dq), installation qualification dure will generate test. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Analytical instrument qualification aiq is the. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. The iq outlines procedures used to confirm the. Us pharmacopeia (usp) general. Instrument Qualification Guidelines.
From www.pdffiller.com
Fillable Online Analytical Instrument Qualification. Calibration and qualification of equipment Instrument Qualification Guidelines The iq outlines procedures used to confirm the. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably. Instrument Qualification Guidelines.
From www.semanticscholar.org
Analytical Instrument Qualification Standardization on the 4Q Model Semantic Scholar Instrument Qualification Guidelines An installation qualification (iq) and an operational qualification (oq). Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Analytical instrument qualification aiq is the. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines The iq outlines procedures used to confirm the. Design qualification (dq), installation qualification dure will generate test. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. I have reviewed. Instrument Qualification Guidelines.
From www.semanticscholar.org
Figure 3 from Overview of Risk‐Based Approach to Phase Appropriate Validation and Instrument Instrument Qualification Guidelines The iq outlines procedures used to confirm the. An installation qualification (iq) and an operational qualification (oq). Design qualification (dq), installation qualification dure will generate test. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. The iq outlines procedures used to confirm the. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in. Instrument Qualification Guidelines.
From pharmablog.in
Qualification of Equipment / Instrument / Utilities SOP PharmaBlog Instrument Qualification Guidelines Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. An installation qualification (iq) and an operational qualification (oq). I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Us pharmacopeia (usp). Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines The iq outlines procedures used to confirm the. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. An installation qualification (iq) and an operational qualification (oq). Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Design qualification (dq), installation qualification. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Analytical instrument qualification aiq is the collection of documented evidence that an. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented in 2008 and remained. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. Analytical instrument qualification. Instrument Qualification Guidelines.
From www.cytivalifesciences.com
Instrument qualification IQ/OQ Cytiva Instrument Qualification Guidelines Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. Design qualification (dq), installation qualification dure will generate test. The iq outlines procedures used to confirm the. I have reviewed key parts. Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. The iq outlines procedures used to confirm the. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. An installation qualification (iq) and an operational qualification (oq). Us pharmacopeia (usp). Instrument Qualification Guidelines.
From www.semanticscholar.org
Figure 1 from Analytical Instrument Qualification Semantic Scholar Instrument Qualification Guidelines Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and document your. The iq outlines procedures used to confirm the. An installation qualification (iq) and an operational qualification (oq). Design qualification (dq), installation qualification dure will generate test. I have reviewed key parts of usp on analytical instrument qualification to highlight areas. Instrument Qualification Guidelines.
From www.slideserve.com
PPT Quality Assurance and Regulatory Compliance for Pharmaceutical Product PowerPoint Instrument Qualification Guidelines I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. Design qualification (dq), installation qualification dure will generate test. The iq outlines procedures used to confirm the. Installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv). Instrument Qualification Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Related Warning Letters Instrument Qualification Guidelines Installation qualification (iq) is the documented collection of activities necessary to establish that an instrument is delivered as designed and. An installation qualification (iq) and an operational qualification (oq). I have reviewed key parts of usp on analytical instrument qualification to highlight areas in the general chapter where care will need to be taken to prevent an. The iq outlines. Instrument Qualification Guidelines.