Bromine Cyclohexene Formula . The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Electrophilic addition of bromine to cyclohexene. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Add bromine solution to both. Cyclohexene reacts with bromine in the same way and under the same conditions as any other. This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene.
from www.chegg.com
Cyclohexene reacts with bromine in the same way and under the same conditions as any other. This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Electrophilic addition of bromine to cyclohexene. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Add bromine solution to both. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene.
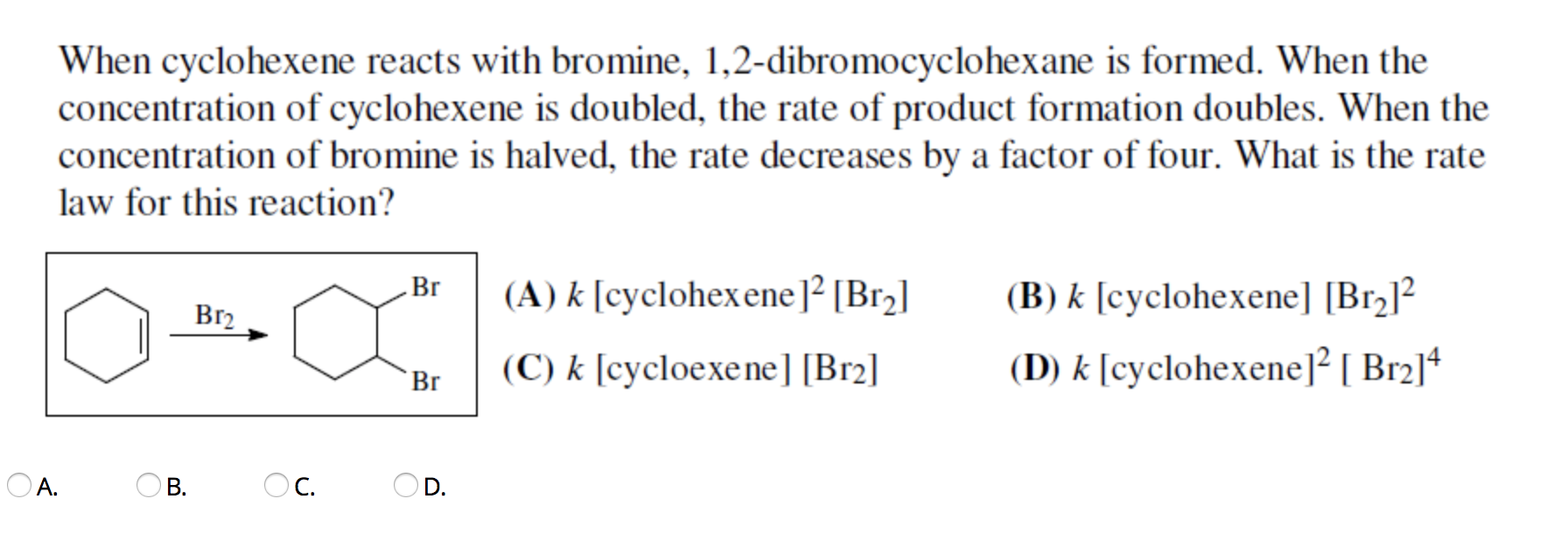
Solved When cyclohexene reacts with bromine,
Bromine Cyclohexene Formula This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Add bromine solution to both. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Cyclohexene reacts with bromine in the same way and under the same conditions as any other. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Electrophilic addition of bromine to cyclohexene. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅.
From www.chemistrystudent.com
Bromination of Benzenes (ALevel) ChemistryStudent Bromine Cyclohexene Formula Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Electrophilic addition of bromine to cyclohexene. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b. Bromine Cyclohexene Formula.
From ar.inspiredpencil.com
Cyclohexene Lewis Structure Bromine Cyclohexene Formula Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Add bromine solution to both. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Cyclohexene reacts with bromine in the same way and under the same conditions as any other. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate. Bromine Cyclohexene Formula.
From ar.inspiredpencil.com
Cyclohexene Bromine Cyclohexene Formula Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Electrophilic addition of bromine to cyclohexene. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Cyclohexene reacts with bromine in the same way. Bromine Cyclohexene Formula.
From alaynameowzavala.blogspot.com
Cyclohexane Reaction With Bromine Bromine Cyclohexene Formula Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Under uv light, brx2 b r x 2 undergoes homolytic. Bromine Cyclohexene Formula.
From www.youtube.com
Testing for an Alkene. Cyclohexene and Bromine waterElectrophilic Bromine Cyclohexene Formula Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Cyclohexene reacts with bromine in the same way and. Bromine Cyclohexene Formula.
From www.fishersci.com
(2Bromoethyl)cyclohexane, 99, Thermo Scientific Chemicals, Quantity Bromine Cyclohexene Formula Add bromine solution to both. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. Cyclohexene reacts with bromine in the same way and under the same conditions as any other. The addition reaction occurs to get reddish bromine. Bromine Cyclohexene Formula.
From www.chegg.com
Solved When cyclohexene reacts with bromine, Bromine Cyclohexene Formula Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Add bromine solution to both. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. This page gives you the facts and a simple, uncluttered mechanism for the. Bromine Cyclohexene Formula.
From www.youtube.com
Bromination of cyclohexene YouTube Bromine Cyclohexene Formula Electrophilic addition of bromine to cyclohexene. Add bromine solution to both. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. This. Bromine Cyclohexene Formula.
From www.numerade.com
SOLVED Show the mechanism for the following reaction conducted at 5 *C Bromine Cyclohexene Formula Electrophilic addition of bromine to cyclohexene. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Under uv light, brx2 b r. Bromine Cyclohexene Formula.
From www.coursehero.com
[Solved] 1. If we do the synthesis reaction of cyclohexene, calculate Bromine Cyclohexene Formula Cyclohexene reacts with bromine in the same way and under the same conditions as any other. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. When bromine is added to the sample, if the reddish color disappear, that means the sample. Bromine Cyclohexene Formula.
From williamdesnhhull.blogspot.com
Reaction of Cyclohexene With Bromine Bromine Cyclohexene Formula The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: When bromine is added to. Bromine Cyclohexene Formula.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Bromine Cyclohexene Formula Add bromine solution to both. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. This page gives you the facts and a simple, uncluttered mechanism for the. Bromine Cyclohexene Formula.
From stock.adobe.com
Stylized molecule model/structural formula of cyclohexene Stock Vector Bromine Cyclohexene Formula Cyclohexene reacts with bromine in the same way and under the same conditions as any other. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full. Bromine Cyclohexene Formula.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Cyclohexene Formula The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Add bromine solution to both. Electrophilic addition of bromine to cyclohexene. This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and. Bromine Cyclohexene Formula.
From chemistry.stackexchange.com
organic chemistry Bromination of cyclohexene in presence of UV light Bromine Cyclohexene Formula Add bromine solution to both. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Cyclohexene reacts with bromine in the same way and under the same conditions as any other. When bromine is added to the sample, if the reddish color. Bromine Cyclohexene Formula.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Bromine Cyclohexene Formula This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. Add bromine solution to both. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Under uv light, brx2 b r x. Bromine Cyclohexene Formula.
From www.numerade.com
SOLVED Discussion In your discussion Write the mechanism for the Bromine Cyclohexene Formula Electrophilic addition of bromine to cyclohexene. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Add bromine solution to both. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark.. Bromine Cyclohexene Formula.
From www.coursehero.com
[Solved] Using line diagrams represents the reaction of cyclohexene Bromine Cyclohexene Formula When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Cyclohexene reacts with bromine in the same way and under the same conditions as any other. Brx2−→hv 2br⋅ b r x 2 → h v. Bromine Cyclohexene Formula.
From www.numerade.com
SOLVED Show the mechanism for the following reaction conducted at 5 Bromine Cyclohexene Formula When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Brx2−→hv 2br⋅ b r x 2 →. Bromine Cyclohexene Formula.
From www.coursehero.com
[Solved] 11. Draw the reaction between cyclohexene and bromine in CCl4 Bromine Cyclohexene Formula Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Cyclohexene reacts with bromine in the same way and under the same conditions as any other.. Bromine Cyclohexene Formula.
From www.coursehero.com
[Solved] 1. In the presence of small amount of bromine under Bromine Cyclohexene Formula This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. The addition reaction occurs to. Bromine Cyclohexene Formula.
From www.numerade.com
SOLVED Consider the mechanism for the reaction of cyclohexene and Bromine Cyclohexene Formula Electrophilic addition of bromine to cyclohexene. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. Brx2−→hv 2br⋅ b r x 2. Bromine Cyclohexene Formula.
From www.chegg.com
Solved 1) When cyclohexene is reacted with Bromine in water, Bromine Cyclohexene Formula Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Electrophilic addition of bromine to cyclohexene. This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. Cyclohexene. Bromine Cyclohexene Formula.
From sielc.com
Cyclohexene SIELC Bromine Cyclohexene Formula The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Electrophilic addition of bromine to cyclohexene. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Cyclohexene reacts with bromine in the same way. Bromine Cyclohexene Formula.
From mavink.com
Cyclohexane Chemical Structure Bromine Cyclohexene Formula Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Electrophilic addition of bromine to cyclohexene. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Add bromine solution to both. This. Bromine Cyclohexene Formula.
From www.numerade.com
SOLVED What is the major product of the reaction between cyclohexene Bromine Cyclohexene Formula Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Add bromine solution to both. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. The addition reaction occurs to get reddish bromine consumed and colorless product formed,. Bromine Cyclohexene Formula.
From saniyahghopdalton.blogspot.com
Reaction of Cyclohexene With Bromine Bromine Cyclohexene Formula Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Add bromine solution to both. This page gives you the facts and a simple,. Bromine Cyclohexene Formula.
From www.transtutors.com
(Solved) In the presence of a small amount of bromine, cyclohexene Bromine Cyclohexene Formula Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Electrophilic addition of bromine to cyclohexene. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Cyclohexene reacts with bromine in the same way and. Bromine Cyclohexene Formula.
From chem.libretexts.org
4.2 Representing ionic and molecular compounds and molecules Bromine Cyclohexene Formula This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the. Add bromine solution to both. Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Cyclohexene reacts with bromine in the same way and under the same conditions as any other. Brx2−→hv 2br⋅ b r. Bromine Cyclohexene Formula.
From www.numerade.com
SOLVED Write a chemical equation for a possible lightcatalyzed Bromine Cyclohexene Formula Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Add bromine solution to both. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Fill 1 cylinder 1/2 full with cyclohexane. Bromine Cyclohexene Formula.
From www.chemistrystudent.com
Bromination of Benzenes (ALevel) ChemistryStudent Bromine Cyclohexene Formula Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. Electrophilic addition of bromine to cyclohexene. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Brx2−→hv 2br⋅ b r x 2 → h v 2 b. Bromine Cyclohexene Formula.
From www.coursehero.com
[Solved] Using line diagrams, represent the reaction of cyclohexene Bromine Cyclohexene Formula Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2 full with cyclohexene. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Electrophilic addition of bromine to cyclohexene. Add bromine solution to both. Cyclohexene reacts with bromine in the same way and under the same conditions as any other. Under. Bromine Cyclohexene Formula.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Bromine Cyclohexene Formula Cyclohexene reacts with bromine in the same way and under the same conditions as any other. Electrophilic addition of bromine to cyclohexene. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so. Bromine Cyclohexene Formula.
From ar.inspiredpencil.com
Cyclohexene Lewis Structure Bromine Cyclohexene Formula Cyclohexene is reacted with bromine in carbon tetrachloride in the dark. Brx2−→hv 2br⋅ b r x 2 → h v 2 b r ⋅. Electrophilic addition of bromine to cyclohexene. Under uv light, brx2 b r x 2 undergoes homolytic splitting to generate br⋅ b r ⋅ radicals: Fill 1 cylinder 1/2 full with cyclohexane and fill the other 1/2. Bromine Cyclohexene Formula.
From byjus.com
Write the molecular formula and structure of cyclohexane, and also give Bromine Cyclohexene Formula The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Add bromine solution to both. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Cyclohexene reacts with bromine in the same way and under the same conditions as any other. Under uv. Bromine Cyclohexene Formula.