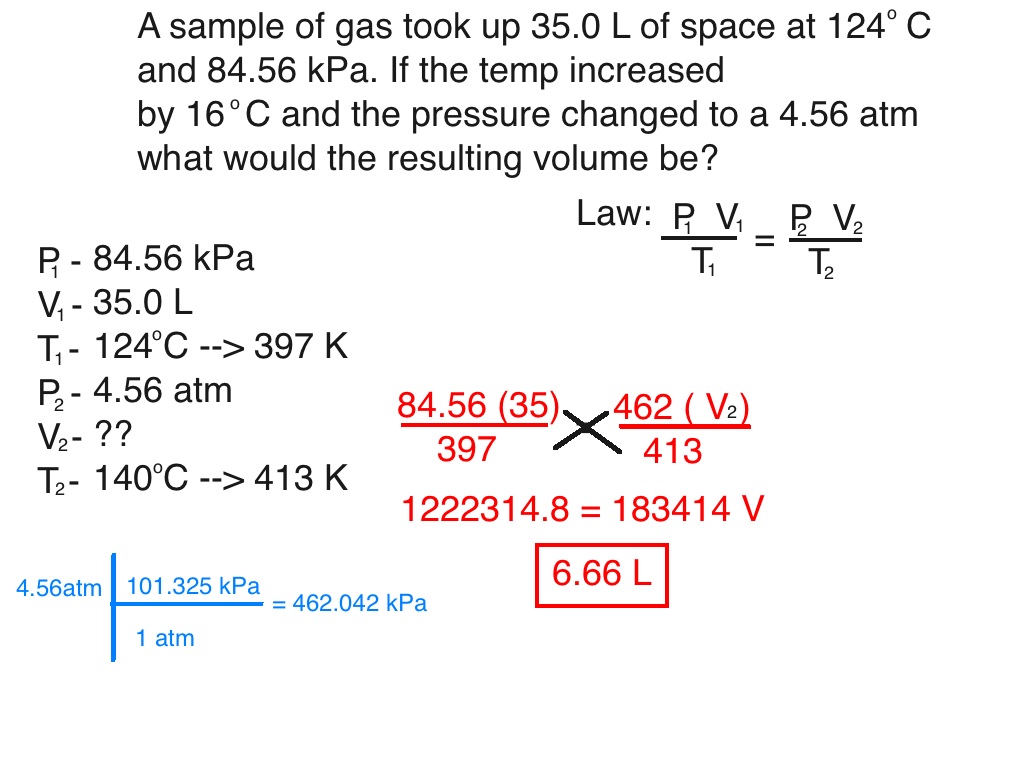
Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve . It is derived from charles' law, boyle's law, and. T = temperature in kelvin. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. See examples of how to use the law to calculate. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Find the value of the gas constant r, the standard. The combined gas law defines the relationship between pressure, temperature, and volume of a gas. Combined gas law can be mathematically expressed as.
from chemistry101efhs.weebly.com
T = temperature in kelvin. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. See examples of how to use the law to calculate. It is derived from charles' law, boyle's law, and. Combined gas law can be mathematically expressed as. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. The combined gas law defines the relationship between pressure, temperature, and volume of a gas. Find the value of the gas constant r, the standard.
Gas Laws Chemistry 101
Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve It is derived from charles' law, boyle's law, and. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Combined gas law can be mathematically expressed as. The combined gas law defines the relationship between pressure, temperature, and volume of a gas. See examples of how to use the law to calculate. Find the value of the gas constant r, the standard. T = temperature in kelvin. It is derived from charles' law, boyle's law, and. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas.
From www.slideserve.com
PPT Chapter 11 Properties of Gases PowerPoint Presentation, free Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Find the value of the gas constant r, the standard. T =. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. See examples of how to use the law to calculate. Find the. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Combined gas law can be mathematically expressed as. Find the value of the gas constant r, the standard. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. T. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Find the value of the gas constant r, the standard. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. The combined gas law defines the relationship between pressure,. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.youtube.com
Using the Combined Gas Law to Solve for T2 YouTube Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve T = temperature in kelvin. Combined gas law can be mathematically expressed as. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Learn how to use the. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From joitksvvp.blob.core.windows.net
Combined Gas Law Formula For V2 at Amy Timmons blog Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. It is derived from charles' law, boyle's law, and. The combined gas law defines the relationship between pressure, temperature, and volume of a gas. Combined gas law can be mathematically expressed as. See examples. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve T = temperature in kelvin. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Learn how to use the combined gas law to. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From studylib.net
The Combined Gas Law How are the Pressure, Volume, and Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. The combined gas law defines the relationship between pressure, temperature, and volume of a gas. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From drcalef.com
The Combined Gas Law Pressure, Volume, and Temperature Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Combined gas law can be mathematically expressed as. T = temperature in kelvin. Find the value of the gas constant r, the standard. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From chemistryskills.com
Ideal Gas Law Combined Gas Law Chemistry Skills Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. The combined gas law defines the relationship between pressure, temperature, and volume of a gas. It is derived from charles' law, boyle's law, and. Find the value of the gas constant r, the standard. Learn how to use the combined. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From sciencenotes.org
Ideal Gas Law Formula and Examples Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve The combined gas law defines the relationship between pressure, temperature, and volume of a gas. It is derived from charles' law, boyle's law, and. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. T = temperature in kelvin. Learn how to use the combined gas law formula. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.youtube.com
Ideal Gas Laws 2 The Combined Gas Equation YouTube Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Combined gas law can be mathematically expressed as. T = temperature in kelvin. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. The. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4199554 Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve See examples of how to use the law to calculate. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Combined gas law can be mathematically expressed as. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID2071846 Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve It is derived from charles' law, boyle's law, and. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the combined gas law to relate the pressure,. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From printablelibmoits.z13.web.core.windows.net
Combined Gas Law Explained Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve T = temperature in kelvin. See examples of how to use the law to calculate. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Combined gas law can be mathematically expressed as. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.youtube.com
CHEMISTRY 101 Combined Gas Law YouTube Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. See examples of how to use the law to calculate. It is derived from charles' law, boyle's law, and. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure,. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.showme.com
Combined Gas Law Problem Fundamental JMC SP2017 Gas Laws Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve See examples of how to use the law to calculate. T = temperature in kelvin. It is derived from charles' law, boyle's law, and. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. The combined gas law defines the relationship between pressure, temperature, and volume of a gas. Learn. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.sliderbase.com
Gas Laws Presentation Chemistry Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve It is derived from charles' law, boyle's law, and. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Learn how to use the combined gas law formula. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.showme.com
When to use combined or ideal gas law ShowMe Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve It is derived from charles' law, boyle's law, and. T = temperature in kelvin. Find the value of the gas constant r, the standard. See examples of how to use the law to calculate. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the combined gas. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID5614600 Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Find the value of the gas constant r, the standard. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. T = temperature in kelvin. See examples of. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.chegg.com
Solved Using The Combined Gas Law Below, Calculate The Vo... Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve The combined gas law defines the relationship between pressure, temperature, and volume of a gas. See examples of how to use the law to calculate. Combined gas law can be mathematically expressed as. Find the value of the gas constant r, the standard. It is derived from charles' law, boyle's law, and. Learn how to use the ideal gas law. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideshare.net
Notes gas laws Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve It is derived from charles' law, boyle's law, and. T = temperature in kelvin. See examples of how to use the law to calculate. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the ideal gas law and the general gas equation to describe the behavior. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Combined gas law can be mathematically expressed as. See examples of how to use the law to calculate. It is derived from charles' law, boyle's law, and. Learn how to use the combined gas law to relate the pressure, volume,. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985 Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Find the value of the gas constant r, the standard. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. T = temperature in kelvin. Learn how to use the combined. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT Gas Law and Gas Behavior PowerPoint Presentation ID3183351 Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve It is derived from charles' law, boyle's law, and. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Find the value of the gas constant r, the standard. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID1414769 Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Find the value of the gas constant r, the standard. Combined gas law can be mathematically expressed as. See examples of how to use the law to calculate. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the ideal gas law and the general gas equation. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.youtube.com
Combined Gas Law (P1V1/T1 = P2V2/T2) Examples, Practice Problems Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve The combined gas law defines the relationship between pressure, temperature, and volume of a gas. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. T = temperature. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From owlcation.com
The Theories and Behavior of Gas Owlcation Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve It is derived from charles' law, boyle's law, and. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Find the value of the gas constant r, the standard. Combined gas law can be mathematically expressed as. T = temperature in kelvin. The combined gas law defines the. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT The Combined and Ideal Gas Laws PowerPoint Presentation, free Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. See examples of how to use the law to calculate. Learn how to use the combined gas law. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.youtube.com
Combined Gas Law Pressure, Volume and Temperature Straight Science Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Find the value of the gas constant r, the standard. The combined gas law defines. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT Ch. 13 Notes Gas Laws PowerPoint Presentation ID6765779 Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve The combined gas law defines the relationship between pressure, temperature, and volume of a gas. It is derived from charles' law, boyle's law, and. T = temperature in kelvin. Find the value of the gas constant r, the standard. Combined gas law can be mathematically expressed as. Learn how to use the combined gas law formula p1v1 t1 = p2v2. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Find the value of the gas constant r, the standard. Learn how to use the ideal gas law and the general gas equation to describe the behavior of a gas. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. It is derived from charles' law, boyle's law,. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Combined gas law can be mathematically expressed as. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. It is derived from charles' law, boyle's. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve It is derived from charles' law, boyle's law, and. See examples of how to use the law to calculate. Learn how to use the combined gas law to relate the pressure, volume, and absolute temperature of a fixed amount of gas. Learn how to use the combined gas law to solve problems involving pressure, volume, temperature and moles of gases.. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.
From boulevarddhonoraire.blogspot.com
Combined Gas Laws Calculator Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve Find the value of the gas constant r, the standard. The combined gas law defines the relationship between pressure, temperature, and volume of a gas. Learn how to use the combined gas law formula p1v1 t1 = p2v2 t2 at constant n to calculate changes in pressure, volume, and temperature of. Learn how to use the ideal gas law and. Should The Combined Gas Law Be Used To Calculate The Pressure In A Bleve.