Glucose-Oxygen Fuel Cell Half Equations . Fuel cells have an important advantage over all. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. The (chemical) energy content of any fuel is called heating. Hydrogen + oxygen → water. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode.
from www.slideserve.com
I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. Fuel cells have an important advantage over all. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Hydrogen + oxygen → water. The (chemical) energy content of any fuel is called heating. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode.
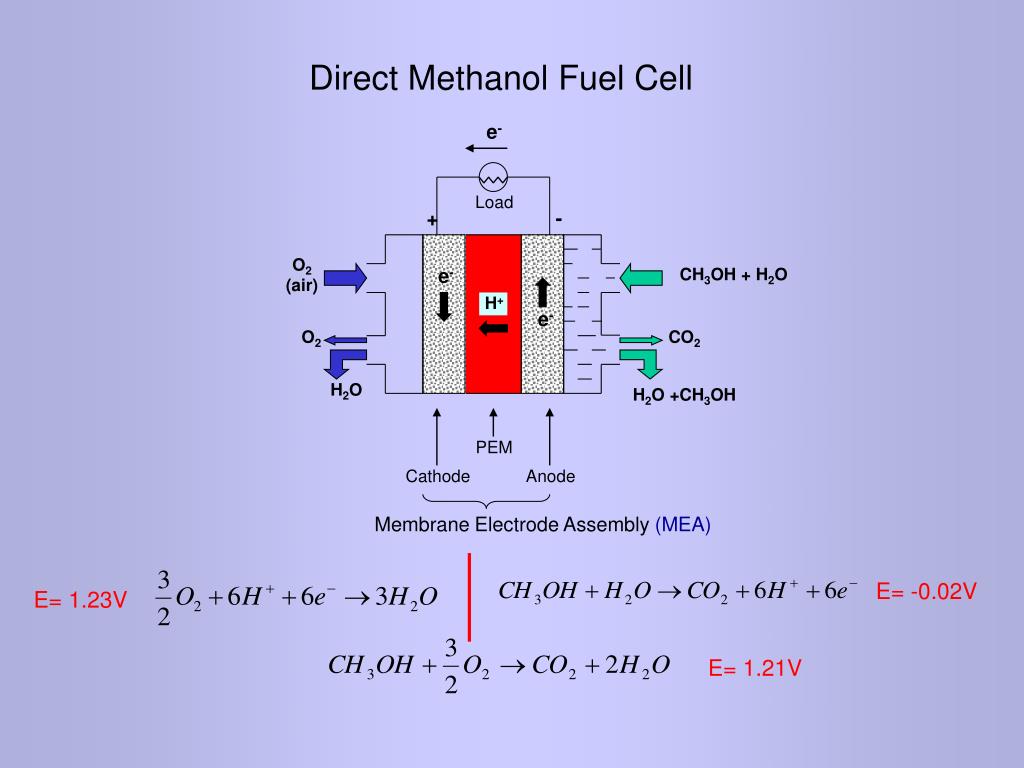
PPT Direct Methanol Fuel Cell Study on anode and cathode catalysts
Glucose-Oxygen Fuel Cell Half Equations The (chemical) energy content of any fuel is called heating. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. Hydrogen + oxygen → water. The (chemical) energy content of any fuel is called heating. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all.
From www.researchgate.net
Schematic representation of the AEMglucose fuel cell. Download Glucose-Oxygen Fuel Cell Half Equations A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. The (chemical) energy content of any fuel is called heating. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. I was given a question recently regarding. Glucose-Oxygen Fuel Cell Half Equations.
From www.numerade.com
SOLVED The theoretical E0cell for the methaneoxygen fuel cell is 1.06 Glucose-Oxygen Fuel Cell Half Equations The (chemical) energy content of any fuel is called heating. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. Hydrogen + oxygen → water. A fuel cell. Glucose-Oxygen Fuel Cell Half Equations.
From quizlet.com
HydrogenOxygen Fuel Cell Diagram Quizlet Glucose-Oxygen Fuel Cell Half Equations I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. Fuel cells have an important advantage over all. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is an electrochemical cell. Glucose-Oxygen Fuel Cell Half Equations.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Glucose-Oxygen Fuel Cell Half Equations A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Hydrogen + oxygen → water. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. Fuel cells have an important advantage over all. The (chemical). Glucose-Oxygen Fuel Cell Half Equations.
From www.slideserve.com
PPT Lecture 3. Fuel Cell Thermodynamics PowerPoint Presentation, free Glucose-Oxygen Fuel Cell Half Equations Hydrogen + oxygen → water. Fuel cells have an important advantage over all. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. The (chemical) energy content of any fuel. Glucose-Oxygen Fuel Cell Half Equations.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Glucose-Oxygen Fuel Cell Half Equations I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen. Glucose-Oxygen Fuel Cell Half Equations.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Glucose-Oxygen Fuel Cell Half Equations When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. Hydrogen + oxygen → water. A fuel cell is an electrochemical cell in which a fuel donates electrons at one. Glucose-Oxygen Fuel Cell Half Equations.
From www.vectorstock.com
Alkaline fuel cell technologies infographic Vector Image Glucose-Oxygen Fuel Cell Half Equations I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. The (chemical) energy content of any fuel is called heating. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Hydrogen + oxygen → water.. Glucose-Oxygen Fuel Cell Half Equations.
From www.semanticscholar.org
Figure 5 from A glucose/O2 fuel cellbased selfpowered biosensor for Glucose-Oxygen Fuel Cell Half Equations A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells have an important advantage over all. The (chemical) energy content. Glucose-Oxygen Fuel Cell Half Equations.
From www.slideserve.com
PPT Direct Methanol Fuel Cell Study on anode and cathode catalysts Glucose-Oxygen Fuel Cell Half Equations A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing. Glucose-Oxygen Fuel Cell Half Equations.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Glucose-Oxygen Fuel Cell Half Equations The (chemical) energy content of any fuel is called heating. Fuel cells have an important advantage over all. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the. Glucose-Oxygen Fuel Cell Half Equations.
From www.mdpi.com
Energies Free FullText Principles and Materials Aspects of Direct Glucose-Oxygen Fuel Cell Half Equations Hydrogen + oxygen → water. Fuel cells have an important advantage over all. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. I was given a. Glucose-Oxygen Fuel Cell Half Equations.
From www.savemyexams.com
Fuel Cells SL IB Chemistry Revision Notes 2025 Glucose-Oxygen Fuel Cell Half Equations Fuel cells have an important advantage over all. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. A fuel cell is an electrochemical cell in which a fuel donates. Glucose-Oxygen Fuel Cell Half Equations.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Glucose-Oxygen Fuel Cell Half Equations A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. The (chemical) energy content. Glucose-Oxygen Fuel Cell Half Equations.
From www.numerade.com
SOLVED Describe a fuel cell. Include equations for the anode and Glucose-Oxygen Fuel Cell Half Equations The (chemical) energy content of any fuel is called heating. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. When the. Glucose-Oxygen Fuel Cell Half Equations.
From www.researchgate.net
Schematic diagram of the glucosepowered fuel cell based on 3D Glucose-Oxygen Fuel Cell Half Equations Fuel cells have an important advantage over all. Hydrogen + oxygen → water. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. When the cell operates, the. Glucose-Oxygen Fuel Cell Half Equations.
From exyguxgbv.blob.core.windows.net
Role Of Catalyst In Fuel Cell at Dennis Pilla blog Glucose-Oxygen Fuel Cell Half Equations When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Hydrogen + oxygen → water. I was given a question recently regarding an oxygen glucose. Glucose-Oxygen Fuel Cell Half Equations.
From www.semanticscholar.org
Figure 2 from A glucose/O2 fuel cellbased selfpowered biosensor for Glucose-Oxygen Fuel Cell Half Equations I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons. Glucose-Oxygen Fuel Cell Half Equations.
From www.researchgate.net
6. Hydrogenoxygen fuel cell scheme. Download Scientific Diagram Glucose-Oxygen Fuel Cell Half Equations The (chemical) energy content of any fuel is called heating. Hydrogen + oxygen → water. Fuel cells have an important advantage over all. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon. Glucose-Oxygen Fuel Cell Half Equations.
From www.slideserve.com
PPT Fuel Cell Catalysts Based on Metal Nanoparticles PowerPoint Glucose-Oxygen Fuel Cell Half Equations Fuel cells have an important advantage over all. Hydrogen + oxygen → water. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. The (chemical) energy content of any fuel is called heating. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water. Glucose-Oxygen Fuel Cell Half Equations.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Glucose-Oxygen Fuel Cell Half Equations When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells have an important advantage over all. Hydrogen + oxygen → water. A fuel. Glucose-Oxygen Fuel Cell Half Equations.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Glucose-Oxygen Fuel Cell Half Equations I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode. Glucose-Oxygen Fuel Cell Half Equations.
From www.tessshebaylo.com
Balanced Equation For Corrosion Of Iron Tessshebaylo Glucose-Oxygen Fuel Cell Half Equations Hydrogen + oxygen → water. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The (chemical) energy content of any fuel is called heating. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at. Glucose-Oxygen Fuel Cell Half Equations.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Glucose-Oxygen Fuel Cell Half Equations A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. The (chemical) energy content of any fuel is called heating. Hydrogen + oxygen → water.. Glucose-Oxygen Fuel Cell Half Equations.
From www.teachoo.com
[Bio] Different ways in which glucose is oxidized to provide energy Glucose-Oxygen Fuel Cell Half Equations The (chemical) energy content of any fuel is called heating. Hydrogen + oxygen → water. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. Fuel cells have an important advantage over all. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode. Glucose-Oxygen Fuel Cell Half Equations.
From chemistryguru.com.sg
2020 P1 Q27 Hydrogen Oxygen Fuel Cell in Alkaline Medium Glucose-Oxygen Fuel Cell Half Equations Hydrogen + oxygen → water. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one. Glucose-Oxygen Fuel Cell Half Equations.
From www.nagwa.com
Question Video Combining Equations to Give the Overall Reaction for a Glucose-Oxygen Fuel Cell Half Equations When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Hydrogen + oxygen → water. When the cell operates, the glucose (c6h1206) molecules react with water at. Glucose-Oxygen Fuel Cell Half Equations.
From www.youtube.com
OCR C6 Fuel Cells (Higher) YouTube Glucose-Oxygen Fuel Cell Half Equations A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. The (chemical) energy content of any fuel is called heating. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. Fuel cells have an important. Glucose-Oxygen Fuel Cell Half Equations.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions Glucose-Oxygen Fuel Cell Half Equations The (chemical) energy content of any fuel is called heating. Hydrogen + oxygen → water. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. Fuel cells have. Glucose-Oxygen Fuel Cell Half Equations.
From brainly.in
Glucose+oxygen=carbon dioxide+water+energy equation Brainly.in Glucose-Oxygen Fuel Cell Half Equations Hydrogen + oxygen → water. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. A fuel cell like this will continue to operate and produce electrical energy as long. Glucose-Oxygen Fuel Cell Half Equations.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip Glucose-Oxygen Fuel Cell Half Equations I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and. Glucose-Oxygen Fuel Cell Half Equations.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Glucose-Oxygen Fuel Cell Half Equations When the cell operates, the glucose (c6h1206) molecules react with water at the negative electrode to form carbon dioxide and hydrogen. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. Hydrogen + oxygen → water. A fuel cell is an electrochemical cell in which a fuel donates electrons. Glucose-Oxygen Fuel Cell Half Equations.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Glucose-Oxygen Fuel Cell Half Equations A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. When the cell operates, the glucose (c 6 h 12 o 6). Glucose-Oxygen Fuel Cell Half Equations.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Glucose-Oxygen Fuel Cell Half Equations When the cell operates, the glucose (c 6 h 12 o 6) molecules react with water at the negative. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen. Glucose-Oxygen Fuel Cell Half Equations.
From ask.learncbse.in
Consider a fuel cell that uses the reaction of ethanol with oxygen to Glucose-Oxygen Fuel Cell Half Equations Fuel cells have an important advantage over all. I was given a question recently regarding an oxygen glucose fuel cell and the question requires writing the half equations for. Hydrogen + oxygen → water. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel. Glucose-Oxygen Fuel Cell Half Equations.