Iec Standards For Medical Devices . This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Iec 60601 series is widely recognized as the basic safety and. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. The base standards for ai in the medical. Several iec standards are now globally recognized by medical device regulators: Requirements for medical electrical equipment and medical electrical systems for medical. Singapore standards (ss) are nationally recognised documents established by consensus and the. Intertek provides testing, certification and training services.
from www.slideserve.com
Requirements for medical electrical equipment and medical electrical systems for medical. Singapore standards (ss) are nationally recognised documents established by consensus and the. The base standards for ai in the medical. Iec 60601 series is widely recognized as the basic safety and. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Intertek provides testing, certification and training services. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Several iec standards are now globally recognized by medical device regulators:
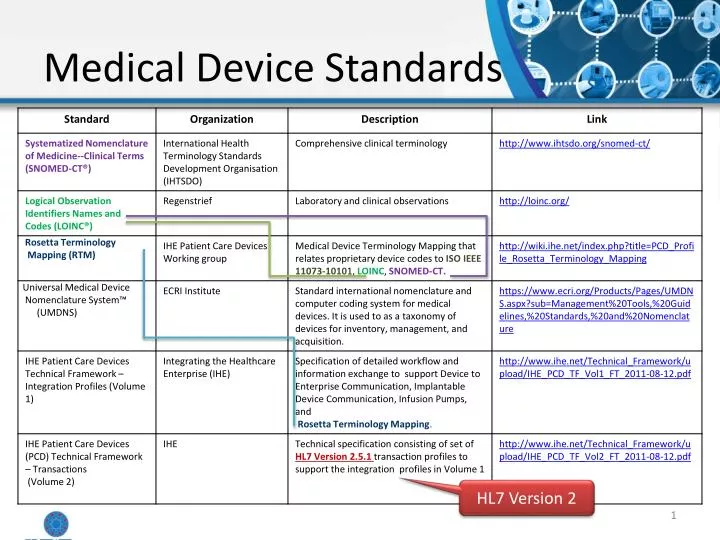
PPT Medical Device Standards PowerPoint Presentation, free download
Iec Standards For Medical Devices Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Several iec standards are now globally recognized by medical device regulators: Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. The base standards for ai in the medical. Requirements for medical electrical equipment and medical electrical systems for medical. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Intertek provides testing, certification and training services. Singapore standards (ss) are nationally recognised documents established by consensus and the. Iec 60601 series is widely recognized as the basic safety and.
From ramtechno.com
Harmonized Standards for Medical Devices and IEC 606011 3.2 Iec Standards For Medical Devices Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. The base standards for ai in the medical. Several iec standards are now globally recognized by medical device regulators: Iec 60601 series is widely recognized as the basic safety and. This standard specifies a process for manufacturers to analyse, specify, develop and. Iec Standards For Medical Devices.
From www.qitubk.com
ANSI AAMI IEC 6236612015 pdf free download Medical devices Part 1 Iec Standards For Medical Devices Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Singapore standards (ss) are nationally recognised documents established by consensus and the. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Iec 60601 series is widely recognized as the basic safety and. This standard. Iec Standards For Medical Devices.
From www.slideserve.com
PPT Calibration and Electrical Safety of Medical Equipment PowerPoint Iec Standards For Medical Devices This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Several iec standards are now globally recognized by medical device regulators: Intertek provides testing, certification and training services. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Singapore standards (ss). Iec Standards For Medical Devices.
From www.qualitymeddev.com
IEC 60601 Overview of the Main Requirements QualityMedDev Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Intertek provides testing, certification and training services. Medical. Iec Standards For Medical Devices.
From templates.rjuuc.edu.np
Iec 62304 Software Development Plan Template Iec Standards For Medical Devices This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Intertek provides testing, certification and training services. Iec 60601 is a series of international standards that specify safety and performance requirements. Iec Standards For Medical Devices.
From www.slideserve.com
PPT Implantable Medical Devices PowerPoint Presentation, free Iec Standards For Medical Devices Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Iec 60601 series is widely recognized as the basic safety and. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Requirements for medical electrical equipment and medical electrical systems for medical. Intertek provides testing,. Iec Standards For Medical Devices.
From www.qualitiso.com
IEC 62304 Medical device Software life cycle Iec Standards For Medical Devices Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Iec 60601 series is widely recognized as the basic safety and. The base standards for ai in the medical. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Several iec standards are now globally. Iec Standards For Medical Devices.
From www.laboratuar.com
IEC/EN 606011 Requirements for Equipment, Basic Safety and Required Iec Standards For Medical Devices The base standards for ai in the medical. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Requirements for medical electrical equipment and medical electrical systems for medical. Intertek provides testing, certification and training services. Several iec standards are now globally recognized by medical device regulators: This standard specifies the basic. Iec Standards For Medical Devices.
From www.sunfiretesting.com
Medical Device IEC 6060112 EMC Testing Lab Sunfire Testing Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. Intertek provides testing, certification and training services. The base standards for ai in the medical. Several iec standards are now globally recognized by medical device regulators: Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. This standard specifies the basic safety. Iec Standards For Medical Devices.
From www.myxxgirl.com
Iec 60601 1 Symbols My XXX Hot Girl Iec Standards For Medical Devices The base standards for ai in the medical. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Requirements for medical electrical equipment and medical electrical systems for medical. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Iec 60601 series is widely recognized. Iec Standards For Medical Devices.
From blog.sierralabs.com
Medical Device Software & SaMDs A Crash Course Iec Standards For Medical Devices This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Singapore standards (ss) are nationally recognised documents established by consensus and the. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. The base standards for ai in the medical.. Iec Standards For Medical Devices.
From www.latticesemi.com
The Importance of Functional Safety Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. Iec 60601 series is widely recognized as the basic safety and. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used. Iec Standards For Medical Devices.
From www.simplexitypd.com
Best Practices IEC 62304 Compliant Medical Device Software Simplexity Iec Standards For Medical Devices Singapore standards (ss) are nationally recognised documents established by consensus and the. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Intertek provides testing, certification and training services. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to.. Iec Standards For Medical Devices.
From studylib.net
IEC 6060112 Medical Devices Top 16 FAQs Iec Standards For Medical Devices This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Several iec standards are now globally recognized by medical device regulators: The base standards for ai in the medical. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. This standard. Iec Standards For Medical Devices.
From www.semanticscholar.org
[PDF] Developing Medical Device Software to be compliant with IEC 62304 Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Iec 60601 series is widely recognized as the basic safety and. The base standards for ai in the medical. Intertek provides testing, certification and training services. This standard specifies a. Iec Standards For Medical Devices.
From www.linkedin.com
IEC 60601 Product Safety Standards for Medical Devices Iec Standards For Medical Devices Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Intertek provides testing, certification and training services. Requirements for medical electrical equipment and medical electrical systems for medical. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. The base standards for ai in the. Iec Standards For Medical Devices.
From www.greenlight.guru
Design Controls For Medical Device Companies [Guide] Iec Standards For Medical Devices The base standards for ai in the medical. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Singapore standards (ss) are nationally recognised documents established by consensus and. Iec Standards For Medical Devices.
From www.parasoft.com
What Is IEC 62304 & How Is It Used in Medical Device Compliance? Iec Standards For Medical Devices Several iec standards are now globally recognized by medical device regulators: This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Intertek provides testing, certification and training services. The base standards for ai in the medical. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability. Iec Standards For Medical Devices.
From www.greenlight.guru
15 Steps to Getting Approval for IEC 606011 Iec Standards For Medical Devices Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Intertek provides testing, certification and training services. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Several iec standards are now globally recognized by medical device regulators: Requirements for medical electrical equipment and medical. Iec Standards For Medical Devices.
From www.element.com
EMC Testing for Medical Electrical Equipment IEC 60601122014 Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. Iec 60601 series is widely recognized as the basic safety and. The base standards for ai in the medical. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Iec 60601 is a series of international standards that specify safety. Iec Standards For Medical Devices.
From oanhthai.com
Top 16 iso 62304 medical device software in 2022 Oanhthai Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. Iec 60601 series is widely recognized as the basic safety and. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Several iec standards. Iec Standards For Medical Devices.
From www.tuleap.org
IEC 62304 the requirements of this Medical Device Software standard Iec Standards For Medical Devices Iec 60601 series is widely recognized as the basic safety and. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Intertek provides testing, certification and training services. The base standards for ai in the medical. Iec 60601 is a series of international standards that specify safety and performance requirements for. Iec Standards For Medical Devices.
From blog.sierralabs.com
A Simple Breakdown of ISO and IEC Standards for SaMD Iec Standards For Medical Devices This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Iec 60601 series is widely recognized as the basic safety and. The base standards for ai in the medical. Iec 60601 is a series. Iec Standards For Medical Devices.
From www.tuvsud.com
IEC 61508 Functional Safety Compliance Certification TÜV SÜD PSB Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. The base standards for ai in the medical. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. This standard. Iec Standards For Medical Devices.
From www.linkedin.com
Safety Standards for Home Healthcare Devices (IEC 60601111) and Their Iec Standards For Medical Devices This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Singapore standards (ss) are nationally recognised documents established by consensus and the. The base standards for ai in the medical. Several iec standards are now globally recognized by medical device regulators: Medical standards are written by around 20. Iec Standards For Medical Devices.
From blog.se.com
IEC vs ANSI Switchgear Understanding the Global Standards Schneider Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. Iec 60601 series is widely recognized as the basic safety and. The base standards for ai in the medical. Intertek provides testing, certification and training services. This standard specifies a process. Iec Standards For Medical Devices.
From www.edn.com
Designing medical devices for isolation and safety EDN Iec Standards For Medical Devices Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Intertek provides testing, certification and training services. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Iec 60601 is a series of international standards that specify safety and performance requirements. Iec Standards For Medical Devices.
From www.youtube.com
Medical Device Usability Highlights of European Regulations and the Iec Standards For Medical Devices Intertek provides testing, certification and training services. Iec 60601 series is widely recognized as the basic safety and. Requirements for medical electrical equipment and medical electrical systems for medical. The base standards for ai in the medical. Singapore standards (ss) are nationally recognised documents established by consensus and the. Iec 60601 is a series of international standards that specify safety. Iec Standards For Medical Devices.
From www.tuv.com
IEC 606011 Medical Electrical Equipment HU TÜV Rheinland Iec Standards For Medical Devices Singapore standards (ss) are nationally recognised documents established by consensus and the. Several iec standards are now globally recognized by medical device regulators: This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. This standard specifies the basic safety and essential performance of medical electrical equipment and systems. Iec Standards For Medical Devices.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Intertek provides testing, certification and training services. The base standards for ai in the medical. Iec 60601 series is widely recognized as the basic safety and. This standard specifies a. Iec Standards For Medical Devices.
From www.greenlight.guru
How to Apply IEC 62304 Requirements for Medical Device Software Iec Standards For Medical Devices Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. The base standards for ai in the medical. Singapore standards (ss) are nationally recognised documents established by consensus and the. Intertek provides testing, certification and training services. Iec 60601 is a series of international standards that specify safety and performance requirements for medical. Iec Standards For Medical Devices.
From www.complianceonline.com
IEC 623042015 Medical Device Software Iec Standards For Medical Devices Requirements for medical electrical equipment and medical electrical systems for medical. The base standards for ai in the medical. This standard specifies the basic safety and essential performance of medical electrical equipment and systems used in the home. Several iec standards are now globally recognized by medical device regulators: Intertek provides testing, certification and training services. Iec 60601 series is. Iec Standards For Medical Devices.
From www.tuleap.org
IEC 62304 standard for medical device software What do you risk if Iec Standards For Medical Devices Singapore standards (ss) are nationally recognised documents established by consensus and the. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Medical standards are written by around 20 technical committees which include iec tc 62 and iso/tc 210. Iec 60601 is a series of international standards that. Iec Standards For Medical Devices.
From www.linkedin.com
TS Quality & Engineering on LinkedIn iec62336 medicaldevices Iec Standards For Medical Devices Iec 60601 series is widely recognized as the basic safety and. Requirements for medical electrical equipment and medical electrical systems for medical. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Several iec standards are now globally recognized by medical device regulators: The base standards for ai. Iec Standards For Medical Devices.
From www.bartolozzi.it
IEC 82304 Medical device use requirements Iec Standards For Medical Devices Intertek provides testing, certification and training services. Iec 60601 series is widely recognized as the basic safety and. This standard specifies a process for manufacturers to analyse, specify, develop and evaluate the usability of medical devices as it relates to. Iec 60601 is a series of international standards that specify safety and performance requirements for medical electrical equipment. The base. Iec Standards For Medical Devices.