Meaning Of Freezing Point Depression . So, the freezing point depression. The freezing point of a solution is less than the freezing point of the pure solvent. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. It is a colligative property of solutions that is generally proportional to. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. This means that a solution must be cooled to a lower temperature than the. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a.
from www.chegg.com
The freezing point of a solution is less than the freezing point of the pure solvent. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. So, the freezing point depression. It is a colligative property of solutions that is generally proportional to. This means that a solution must be cooled to a lower temperature than the. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity.
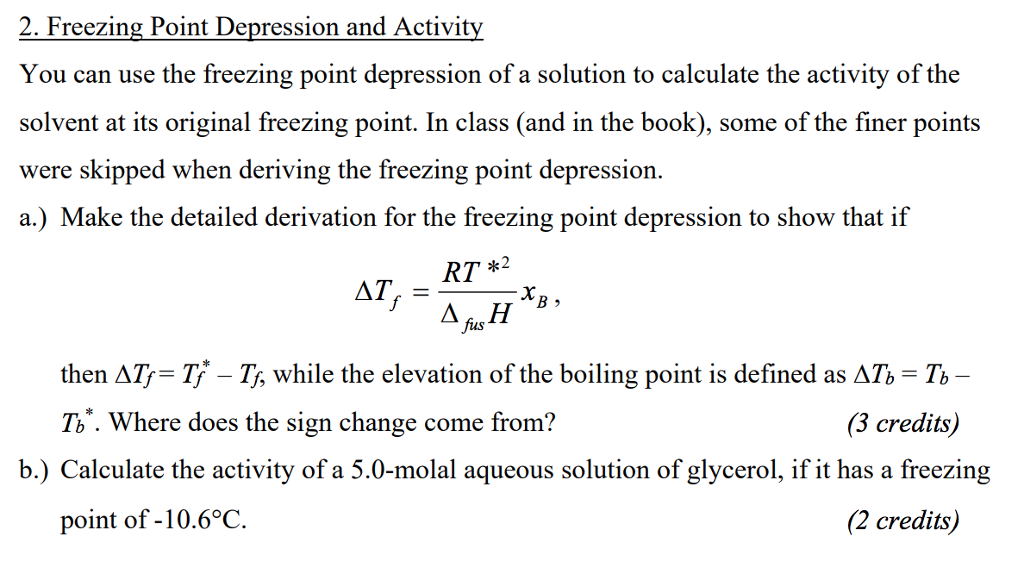
2. Freezing Point Depression and Activit You can use
Meaning Of Freezing Point Depression It is a colligative property of solutions that is generally proportional to. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. So, the freezing point depression. It is a colligative property of solutions that is generally proportional to. The freezing point of a solution is less than the freezing point of the pure solvent. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. This means that a solution must be cooled to a lower temperature than the.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation ID3199294 Meaning Of Freezing Point Depression So, the freezing point depression. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. The freezing point of a solution is less than the freezing point. Meaning Of Freezing Point Depression.
From loeaanczy.blob.core.windows.net
Chlorine Boiling Point And Melting Point at Jane Gibbs blog Meaning Of Freezing Point Depression So, the freezing point depression. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. The freezing point of a solution is less than the freezing point. Meaning Of Freezing Point Depression.
From www.numerade.com
SOLVED Constants for freezingpoint depression and boilingpoint Meaning Of Freezing Point Depression Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. It is a colligative property of solutions that is generally proportional to. Freezing point. Meaning Of Freezing Point Depression.
From facts.net
12 Extraordinary Facts About Freezing Point Depression Meaning Of Freezing Point Depression This means that a solution must be cooled to a lower temperature than the. It is a colligative property of solutions that is generally proportional to. The freezing point of a solution is less than the freezing point of the pure solvent. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes.. Meaning Of Freezing Point Depression.
From pdfprof.com
colligative properties freezing point depression and molar mass Meaning Of Freezing Point Depression This means that a solution must be cooled to a lower temperature than the. The freezing point of a solution is less than the freezing point of the pure solvent. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. Freezing point depression is a. Meaning Of Freezing Point Depression.
From www.science-sparks.com
What is freezing point depression? Meaning Of Freezing Point Depression The freezing point of a solution is less than the freezing point of the pure solvent. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. This means that a solution must be cooled to a lower temperature than the. Freezing point depression refers to. Meaning Of Freezing Point Depression.
From ar.inspiredpencil.com
Freezing Point Depression Constant Meaning Of Freezing Point Depression It is a colligative property of solutions that is generally proportional to. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. The freezing. Meaning Of Freezing Point Depression.
From ar.inspiredpencil.com
Freezing Point Depression Constant Meaning Of Freezing Point Depression So, the freezing point depression. This means that a solution must be cooled to a lower temperature than the. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid,. Meaning Of Freezing Point Depression.
From chemistnotes.com
Depression of Freezing Point Equation, Definition, and Applications Meaning Of Freezing Point Depression Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. It is a colligative property of solutions that is generally proportional to. So, the freezing point depression. This means that a solution must be cooled to a lower temperature than the. The freezing point of a solution is less than the freezing. Meaning Of Freezing Point Depression.
From slideplayer.com
Chapter 16 Mixtures & Solutions ppt download Meaning Of Freezing Point Depression This means that a solution must be cooled to a lower temperature than the. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. So, the freezing point depression. It is a colligative property of solutions that is generally proportional to. Freezing point depression is a colligative property observed. Meaning Of Freezing Point Depression.
From www.slideserve.com
PPT Solutions and Their Properties PowerPoint Presentation, free Meaning Of Freezing Point Depression Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. This means that a solution must be cooled to a lower temperature than the. The freezing point of a solution is less than the freezing point of the pure solvent. So, the freezing point depression. Freezing point depression refers. Meaning Of Freezing Point Depression.
From brothersontech.com
Applications Of Freezing Point Depression Meaning Of Freezing Point Depression Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not. Meaning Of Freezing Point Depression.
From www.youtube.com
Freezing Point Depression Explained YouTube Meaning Of Freezing Point Depression It is a colligative property of solutions that is generally proportional to. This means that a solution must be cooled to a lower temperature than the. The freezing point of a solution is less than the freezing point of the pure solvent. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered. Meaning Of Freezing Point Depression.
From www.slideserve.com
PPT Freezing Point Depression PowerPoint Presentation, free download Meaning Of Freezing Point Depression Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. This means that a solution must be cooled to a lower temperature than the. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. Freezing point depression is a phenomenon in. Meaning Of Freezing Point Depression.
From ar.inspiredpencil.com
Freezing Point Depression Constant Meaning Of Freezing Point Depression What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. This means that a solution must be cooled to a lower temperature than the.. Meaning Of Freezing Point Depression.
From coldwiki.blogspot.com
Freezingpoint depression Meaning Of Freezing Point Depression The freezing point of a solution is less than the freezing point of the pure solvent. It is a colligative property of solutions that is generally proportional to. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. Freezing point depression is a colligative property observed in solutions that. Meaning Of Freezing Point Depression.
From www.differencebetween.com
Difference Between Freezing Point Depression and Boiling Point Meaning Of Freezing Point Depression What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. The freezing point of a solution is less than the freezing point of the. Meaning Of Freezing Point Depression.
From www.youtube.com
calculating freezing point depression YouTube Meaning Of Freezing Point Depression Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not. Meaning Of Freezing Point Depression.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer Meaning Of Freezing Point Depression The freezing point of a solution is less than the freezing point of the pure solvent. So, the freezing point depression. This means that a solution must be cooled to a lower temperature than the. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity.. Meaning Of Freezing Point Depression.
From general.chemistrysteps.com
Freezing Point Depression Chemistry Steps Meaning Of Freezing Point Depression Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. So, the freezing point depression. This means that a solution must be cooled to a lower temperature than the. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. It is. Meaning Of Freezing Point Depression.
From www.chegg.com
2. Freezing Point Depression and Activit You can use Meaning Of Freezing Point Depression The freezing point of a solution is less than the freezing point of the pure solvent. This means that a solution must be cooled to a lower temperature than the. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. Freezing point depression refers to the lowering of the. Meaning Of Freezing Point Depression.
From youtube.com
Calculate Freezing Point Depression YouTube Meaning Of Freezing Point Depression The freezing point of a solution is less than the freezing point of the pure solvent. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. So, the freezing point depression. Freezing point depression is a phenomenon in physical chemistry where the freezing point of. Meaning Of Freezing Point Depression.
From sciencenotes.org
Freezing Point Depression Formula and Definition Meaning Of Freezing Point Depression Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. The freezing point of a solution is less than the freezing point of the pure solvent. So, the freezing point depression. It is a colligative property of solutions that is generally proportional to. Freezing point depression refers to the. Meaning Of Freezing Point Depression.
From unacademy.com
Depression of Freezing Point Unacademy Meaning Of Freezing Point Depression So, the freezing point depression. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. This means that a solution must be cooled to a lower temperature than the. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding. Meaning Of Freezing Point Depression.
From www.slideserve.com
PPT Solutions part 2 PowerPoint Presentation ID706322 Meaning Of Freezing Point Depression It is a colligative property of solutions that is generally proportional to. So, the freezing point depression. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on. Meaning Of Freezing Point Depression.
From www.slideserve.com
PPT Unit 12 Properties of Solutions PowerPoint Presentation, free Meaning Of Freezing Point Depression The freezing point of a solution is less than the freezing point of the pure solvent. It is a colligative property of solutions that is generally proportional to. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. So, the freezing point depression. What this means is that the amount of freezing. Meaning Of Freezing Point Depression.
From gamesmartz.com
Freezing Point Depression Definition & Image GameSmartz Meaning Of Freezing Point Depression The freezing point of a solution is less than the freezing point of the pure solvent. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. Freezing point depression is a. Meaning Of Freezing Point Depression.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry Meaning Of Freezing Point Depression The freezing point of a solution is less than the freezing point of the pure solvent. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. Freezing point. Meaning Of Freezing Point Depression.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID478383 Meaning Of Freezing Point Depression What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. So, the freezing point depression. Freezing point depression is a phenomenon in physical chemistry. Meaning Of Freezing Point Depression.
From scienceinfo.com
Depression of Freezing Point Meaning Of Freezing Point Depression Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. So, the freezing point depression. This means that a solution must be cooled to. Meaning Of Freezing Point Depression.
From www.youtube.com
Calculating freezing point depression YouTube Meaning Of Freezing Point Depression What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. So, the freezing point depression. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. Freezing point depression is a colligative property observed in. Meaning Of Freezing Point Depression.
From www.youtube.com
Depression in freezing Point Solution Chemistry animated Meaning Of Freezing Point Depression It is a colligative property of solutions that is generally proportional to. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. Freezing point depression is a phenomenon in physical chemistry where the freezing point of a liquid is lowered by adding a. So, the freezing point depression. The freezing point of. Meaning Of Freezing Point Depression.
From www.slideserve.com
PPT Determining the Molecular Mass by Freezing Point Depression Meaning Of Freezing Point Depression This means that a solution must be cooled to a lower temperature than the. It is a colligative property of solutions that is generally proportional to. The freezing point of a solution is less than the freezing point of the pure solvent. So, the freezing point depression. Freezing point depression refers to the lowering of the freezing point of solvents. Meaning Of Freezing Point Depression.
From sharpsnapper.com
Freezing Point Depression Calculator Find for Different Solvents Meaning Of Freezing Point Depression The freezing point of a solution is less than the freezing point of the pure solvent. What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. This means that a solution must be cooled to a lower temperature than the. Freezing point depression is a. Meaning Of Freezing Point Depression.
From www.slideserve.com
PPT Solutions part 2 PowerPoint Presentation ID706322 Meaning Of Freezing Point Depression What this means is that the amount of freezing point depression depends on how many particles dissolve in the liquid, not on their chemical identity. The freezing point of a solution is less than the freezing point of the pure solvent. This means that a solution must be cooled to a lower temperature than the. Freezing point depression refers to. Meaning Of Freezing Point Depression.