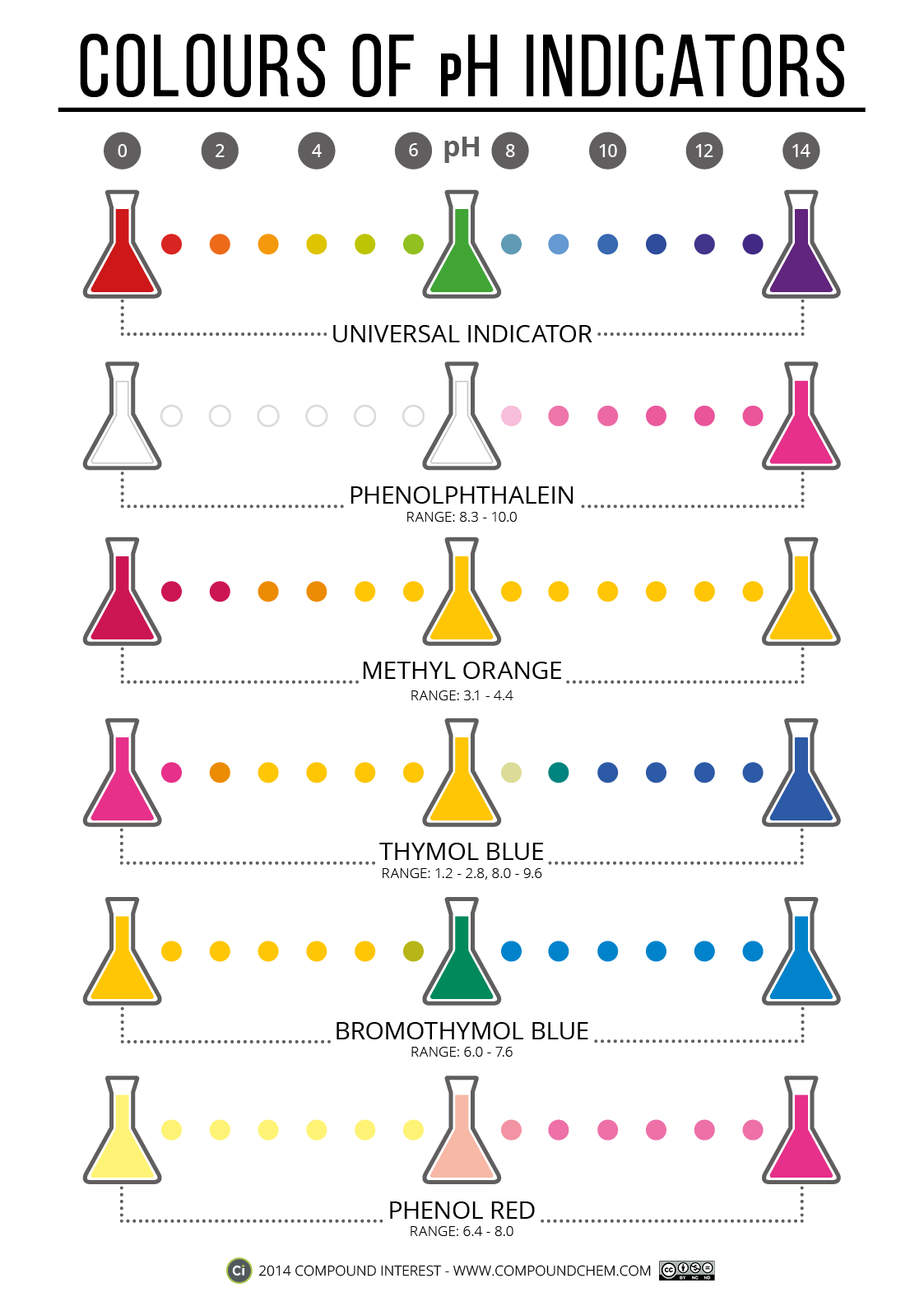
Indicator Of Bases . This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This page assumes that you know about ph curves for all the. When titrating a strong acid or base, the endpoint should occur at a ph of 7. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Indicators are substances whose solutions change color due to changes in ph.
from sites.google.com
This page assumes that you know about ph curves for all the. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. When titrating a strong acid or base, the endpoint should occur at a ph of 7. Indicators are substances whose solutions change color due to changes in ph.
AP Unit 6 Acid Base Equilibria Baumann Chemistry
Indicator Of Bases This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. This page assumes that you know about ph curves for all the. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Indicators are substances whose solutions change color due to changes in ph. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. When titrating a strong acid or base, the endpoint should occur at a ph of 7. This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs.
From www.researchgate.net
Acidbase indicators and their parameters. Download Table Indicator Of Bases Indicators are substances whose solutions change color due to changes in ph. When titrating a strong acid or base, the endpoint should occur at a ph of 7. This page assumes that you know about ph curves for all the. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. This is a charge. Indicator Of Bases.
From pediaa.com
Difference Between Acid Base Indicator and Universal Indicator Indicator Of Bases To be effective, the right indicator must be used that changes color as close to the endpoint as possible. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. Indicators. Indicator Of Bases.
From foundoutaboutchemistry.blogspot.com
Found Out About Chemistry Acidbase indicator charts Indicator Of Bases This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. When titrating a. Indicator Of Bases.
From theory.labster.com
SäureBasenIndikatorTabelle Labster Theory Indicator Of Bases An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This page assumes that you know about ph curves for all the. When titrating a strong acid or base, the endpoint should occur at a ph of 7. To be effective, the right indicator must be used that changes color as close. Indicator Of Bases.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Of Bases Indicators are substances whose solutions change color due to changes in ph. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. To be effective, the right indicator must be used that changes color as close. Indicator Of Bases.
From sites.google.com
AP Unit 6 Acid Base Equilibria Baumann Chemistry Indicator Of Bases We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. Indicators are substances. Indicator Of Bases.
From davidswart.wordpress.com
Acids and Bases The Art of Science Indicator Of Bases This page assumes that you know about ph curves for all the. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Indicator Of Bases.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ Indicator Of Bases An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in.. Indicator Of Bases.
From www.slideserve.com
PPT ACID BASE REACTIONS PowerPoint Presentation, free download ID Indicator Of Bases We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. When titrating a strong acid or base, the endpoint should occur at a ph of 7. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Acid and base indicators are usually used to. Indicator Of Bases.
From www.youtube.com
ACID BASE INDICATORS YouTube Indicator Of Bases They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This page assumes that you know about ph curves for all the. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. An indicator is a weak acid that ionizes. Indicator Of Bases.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Of Bases We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. This page assumes that you know about ph curves for all the. This is a charge of common indicators, an explanation of how. Indicator Of Bases.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Of Bases We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Indicators are. Indicator Of Bases.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6973259 Indicator Of Bases An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. When titrating a strong acid or base, the endpoint should occur at a ph of 7. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. We can. Indicator Of Bases.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Of Bases They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This page assumes that you know about ph curves for all the. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. An indicator is a weak acid. Indicator Of Bases.
From www.slideserve.com
PPT Chapter 5 Acids and Bases PowerPoint Presentation, free download Indicator Of Bases We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. Indicators are substances whose solutions change color due to changes in ph. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Acid and base indicators are usually used to detect the endpoint of. Indicator Of Bases.
From www.youtube.com
Acid Base Indicators Introduction Acids and Bases Chemistry Practice Indicator Of Bases They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. When titrating a strong acid or base, the endpoint should occur at a ph of 7. We can. Indicator Of Bases.
From www.slideserve.com
PPT Acid/Base Indicators PowerPoint Presentation, free download ID Indicator Of Bases We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Indicators are substances whose solutions change color due to changes in ph. Acid and base indicators are usually. Indicator Of Bases.
From chem.libretexts.org
17.3 AcidBase Indicators Chemistry LibreTexts Indicator Of Bases This page assumes that you know about ph curves for all the. Indicators are substances whose solutions change color due to changes in ph. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. We. Indicator Of Bases.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Indicator Of Bases Acid and base indicators are usually used to detect the endpoint of acid and base titrations. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. An indicator. Indicator Of Bases.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free Indicator Of Bases We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about. Indicator Of Bases.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID546616 Indicator Of Bases They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Indicators are substances whose solutions change color due to changes in ph. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. To be effective, the right indicator. Indicator Of Bases.
From www.youtube.com
Acidbase indicators Acids and bases AP Chemistry Khan Academy Indicator Of Bases When titrating a strong acid or base, the endpoint should occur at a ph of 7. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This page assumes that. Indicator Of Bases.
From pt.slideshare.net
Acids & Bases Indicators Indicator Of Bases When titrating a strong acid or base, the endpoint should occur at a ph of 7. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This page assumes that you know about ph curves for all the. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Indicator Of Bases.
From www.slideserve.com
PPT Conjugate acids and bases PowerPoint Presentation, free download Indicator Of Bases When titrating a strong acid or base, the endpoint should occur at a ph of 7. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. An indicator. Indicator Of Bases.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator Of Bases When titrating a strong acid or base, the endpoint should occur at a ph of 7. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This page assumes that you know about ph curves for all the. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Indicator Of Bases.
From www.researchgate.net
Characteristics of the acidbase indicators Download Table Indicator Of Bases They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Indicators are substances whose solutions change color due to changes in ph. An indicator is a weak. Indicator Of Bases.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 Indicator Of Bases This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. To be effective, the right indicator must be used that changes color as close to the endpoint as possible. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or. Indicator Of Bases.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5741622 Indicator Of Bases To be effective, the right indicator must be used that changes color as close to the endpoint as possible. This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. This page assumes that you know about ph curves for all the. Indicators are substances whose solutions change. Indicator Of Bases.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo Indicator Of Bases Acid and base indicators are usually used to detect the endpoint of acid and base titrations. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. When titrating a strong acid or base, the endpoint should occur at a ph of 7. They are usually weak acids or bases, but their conjugate. Indicator Of Bases.
From foundoutaboutchemistry.blogspot.com
Found Out About Chemistry Acidbase indicator charts Indicator Of Bases To be effective, the right indicator must be used that changes color as close to the endpoint as possible. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. When titrating a strong acid or base, the endpoint should occur at a ph of 7. They are usually weak acids or bases,. Indicator Of Bases.
From www.slideserve.com
PPT PROPERTIES OF ACIDS & BASES PowerPoint Presentation, free Indicator Of Bases This page assumes that you know about ph curves for all the. When titrating a strong acid or base, the endpoint should occur at a ph of 7. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. An indicator is a weak acid that ionizes within a known ph range, usually about 2. Indicator Of Bases.
From www.scribd.com
ACIDBASE INDICATORS 211.ppt Acid Ph Indicator Of Bases An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. When titrating. Indicator Of Bases.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Indicator Of Bases Indicators are substances whose solutions change color due to changes in ph. When titrating a strong acid or base, the endpoint should occur at a ph of 7. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are usually weak acids or bases, but their conjugate base or acid forms. Indicator Of Bases.
From www.slideserve.com
PPT Acid, Base, Universal Indicator and PH Scale PowerPoint Indicator Of Bases This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. Indicators are substances whose solutions change color due to changes in ph. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. When titrating a strong acid or base, the endpoint. Indicator Of Bases.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Indicator Of Bases Acid and base indicators are usually used to detect the endpoint of acid and base titrations. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the deprotonated form as \(\ce{in. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This page. Indicator Of Bases.