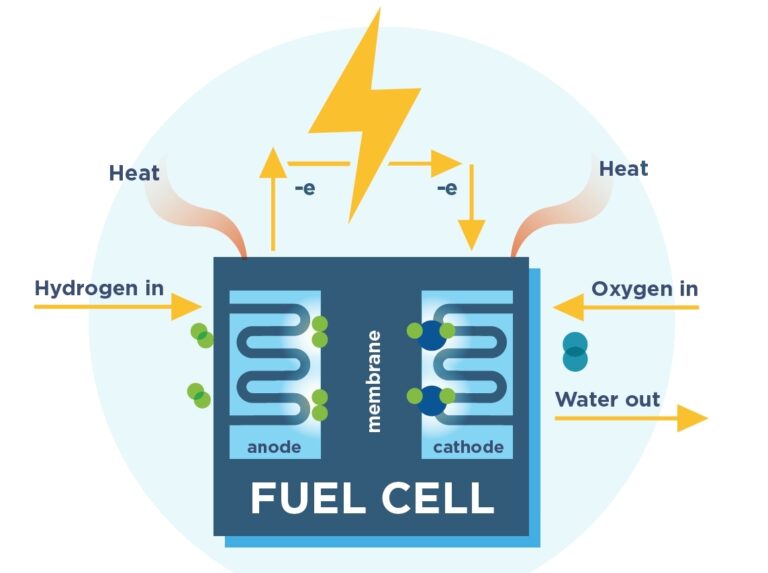
Half Equation For Hydrogen Fuel Cells . We can break down this overall reaction into two half. O 2 + 2h 2 o. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. The fuel is added to the cell and then there is. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. It has a cell potential of 0.00v, measured. The (chemical) energy content of any fuel is.
from www.linquip.com
revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. O 2 + 2h 2 o. The fuel is added to the cell and then there is. It has a cell potential of 0.00v, measured. The (chemical) energy content of any fuel is. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. We can break down this overall reaction into two half. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water.
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip
Half Equation For Hydrogen Fuel Cells The (chemical) energy content of any fuel is. We can break down this overall reaction into two half. The fuel is added to the cell and then there is. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. The (chemical) energy content of any fuel is. O 2 + 2h 2 o. It has a cell potential of 0.00v, measured. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the.
From chemistryguru.com.sg
2020 P1 Q27 Hydrogen Oxygen Fuel Cell in Alkaline Medium Half Equation For Hydrogen Fuel Cells The (chemical) energy content of any fuel is. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. O 2 + 2h 2 o. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. We can break down this overall reaction into two half.. Half Equation For Hydrogen Fuel Cells.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Half Equation For Hydrogen Fuel Cells O 2 + 2h 2 o. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. We can break down this overall reaction into two half. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. a somewhat oversimplified diagram of a fuel cell. Half Equation For Hydrogen Fuel Cells.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Half Equation For Hydrogen Fuel Cells We can break down this overall reaction into two half. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. fuel cells produce electrical energy. Half Equation For Hydrogen Fuel Cells.
From chem.libretexts.org
2.2. Hydrogen, The Simplest Atom Chemistry LibreTexts Half Equation For Hydrogen Fuel Cells It has a cell potential of 0.00v, measured. We can break down this overall reaction into two half. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. The fuel is added to the cell and then there is. O 2 + 2h 2 o. a somewhat oversimplified diagram of a fuel. Half Equation For Hydrogen Fuel Cells.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Half Equation For Hydrogen Fuel Cells The (chemical) energy content of any fuel is. O 2 + 2h 2 o. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. We can break down this overall reaction into two half. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of. Half Equation For Hydrogen Fuel Cells.
From www.numerade.com
SOLVED Describe a fuel cell. Include equations for the anode and Half Equation For Hydrogen Fuel Cells the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. The fuel is added to the cell and then there is. O 2 + 2h 2 o. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. It has a cell potential of 0.00v,. Half Equation For Hydrogen Fuel Cells.
From www.nagwa.com
Question Video Combining Equations to Give the Overall Reaction for a Half Equation For Hydrogen Fuel Cells O 2 + 2h 2 o. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. The fuel is added to the cell and then there is. The (chemical) energy content of any fuel is. the overall reaction in a hydrogen fuel. Half Equation For Hydrogen Fuel Cells.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Half Equation For Hydrogen Fuel Cells revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. The fuel is added to the cell and then there is. It has a cell potential of 0.00v, measured. The (chemical) energy content of any fuel is. fuel cells produce electrical energy using a reaction between an external fuel source. Half Equation For Hydrogen Fuel Cells.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip Half Equation For Hydrogen Fuel Cells fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The fuel is added to the cell and then there is. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. It has a cell potential of 0.00v, measured. a somewhat oversimplified diagram of. Half Equation For Hydrogen Fuel Cells.
From www.vectorstock.com
Alkaline fuel cell technologies infographic Vector Image Half Equation For Hydrogen Fuel Cells O 2 + 2h 2 o. The fuel is added to the cell and then there is. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. fuel cells produce electrical energy using. Half Equation For Hydrogen Fuel Cells.
From www.vrogue.co
Solved Methanol Fuel Cells Use The Following Reaction vrogue.co Half Equation For Hydrogen Fuel Cells revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. It has a cell potential of 0.00v, measured. The fuel is added to the cell and then there is. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The (chemical) energy content. Half Equation For Hydrogen Fuel Cells.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions Half Equation For Hydrogen Fuel Cells the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. It has a cell potential of 0.00v, measured. We can break down this overall reaction into. Half Equation For Hydrogen Fuel Cells.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Half Equation For Hydrogen Fuel Cells a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. O 2 + 2h 2 o. The (chemical) energy content of any fuel is. We can break down this overall reaction into two half. the overall reaction in a hydrogen fuel cell. Half Equation For Hydrogen Fuel Cells.
From www.iasparliament.com
Hydrogen The Fuel of the Future GS III G.S III Environment Half Equation For Hydrogen Fuel Cells The fuel is added to the cell and then there is. The (chemical) energy content of any fuel is. O 2 + 2h 2 o. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen). Half Equation For Hydrogen Fuel Cells.
From www.chegg.com
Solved Hydrogen Fuel Cell Fuel cells are electrochemical Half Equation For Hydrogen Fuel Cells The fuel is added to the cell and then there is. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. The (chemical) energy content of any fuel is. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and. Half Equation For Hydrogen Fuel Cells.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Half Equation For Hydrogen Fuel Cells revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. We can break down this overall reaction into two half. The (chemical) energy content of any fuel is. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. a somewhat oversimplified diagram of. Half Equation For Hydrogen Fuel Cells.
From www.slideserve.com
PPT Fuel Cell Catalysts Based on Metal Nanoparticles PowerPoint Half Equation For Hydrogen Fuel Cells The fuel is added to the cell and then there is. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. the overall reaction. Half Equation For Hydrogen Fuel Cells.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Half Equation For Hydrogen Fuel Cells O 2 + 2h 2 o. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. The (chemical) energy content of any fuel is. The fuel is added to the cell and then there is. We can break down this overall reaction into two half. It has a cell potential of 0.00v, measured.. Half Equation For Hydrogen Fuel Cells.
From www.tessshebaylo.com
What Is The Half Equation For Hydrogen Tessshebaylo Half Equation For Hydrogen Fuel Cells O 2 + 2h 2 o. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The (chemical) energy content of any fuel is. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. revision notes on electrode reactions in hydrogen fuel cells for. Half Equation For Hydrogen Fuel Cells.
From www.kipost.net
전기차용 배터리 소재, 더 멀리...더 저렴하게 Half Equation For Hydrogen Fuel Cells The fuel is added to the cell and then there is. O 2 + 2h 2 o. The (chemical) energy content of any fuel is. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the. Half Equation For Hydrogen Fuel Cells.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Half Equation For Hydrogen Fuel Cells It has a cell potential of 0.00v, measured. The (chemical) energy content of any fuel is. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. We can break down this overall reaction into two half. O 2 + 2h 2 o. fuel cells produce electrical energy using a reaction between an. Half Equation For Hydrogen Fuel Cells.
From brainly.com
write an ionic half equation for the formation of hydrogen during this Half Equation For Hydrogen Fuel Cells The fuel is added to the cell and then there is. It has a cell potential of 0.00v, measured. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. We can break down this overall reaction into two half. a somewhat oversimplified diagram of a fuel cell in which the cell reaction. Half Equation For Hydrogen Fuel Cells.
From www.youtube.com
OCR C6 Fuel Cells (Higher) YouTube Half Equation For Hydrogen Fuel Cells We can break down this overall reaction into two half. O 2 + 2h 2 o. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. The fuel is added to the cell and then there is. fuel cells produce electrical energy. Half Equation For Hydrogen Fuel Cells.
From www.gauthmath.com
Solved = Hydrogen is used in fuel cells. In a fuel cell with an Half Equation For Hydrogen Fuel Cells We can break down this overall reaction into two half. It has a cell potential of 0.00v, measured. The fuel is added to the cell and then there is. The (chemical) energy content of any fuel is. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. O 2 + 2h 2. Half Equation For Hydrogen Fuel Cells.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Half Equation For Hydrogen Fuel Cells fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. We can break down this overall reaction into two half. O 2 + 2h 2 o. The (chemical) energy content of any fuel is. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of. Half Equation For Hydrogen Fuel Cells.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Half Equation For Hydrogen Fuel Cells It has a cell potential of 0.00v, measured. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. The fuel is added to the. Half Equation For Hydrogen Fuel Cells.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Half Equation For Hydrogen Fuel Cells the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. The fuel is added to the cell and then there is. revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. It has a cell potential of 0.00v, measured. fuel cells produce electrical. Half Equation For Hydrogen Fuel Cells.
From openclipart.org
Clipart Direct Methanol Fuel Cell Half Equation For Hydrogen Fuel Cells a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. It has a cell potential of 0.00v, measured. the overall reaction in a hydrogen. Half Equation For Hydrogen Fuel Cells.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Half Equation For Hydrogen Fuel Cells the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. The (chemical) energy content of any fuel is. O 2 + 2h 2 o. We can. Half Equation For Hydrogen Fuel Cells.
From exolmxiop.blob.core.windows.net
Hydrogen Fuel Cells Generate Electricity By Combining Hydrogen With Half Equation For Hydrogen Fuel Cells We can break down this overall reaction into two half. It has a cell potential of 0.00v, measured. The fuel is added to the cell and then there is. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. a somewhat oversimplified diagram of a fuel cell in which the cell reaction. Half Equation For Hydrogen Fuel Cells.
From www.slideshare.net
Hydrogen fuel cells Half Equation For Hydrogen Fuel Cells revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. The fuel is added to the cell and then there is. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. We can break. Half Equation For Hydrogen Fuel Cells.
From large.stanford.edu
Hydrogen Fuel Cells Half Equation For Hydrogen Fuel Cells revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the. We can break down this overall reaction into two half. It has a cell potential of 0.00v, measured. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. The (chemical) energy content of any. Half Equation For Hydrogen Fuel Cells.
From www.savemyexams.com
Fuel Cells HL IB Chemistry Revision Notes 2025 Save My Exams Half Equation For Hydrogen Fuel Cells a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen). Half Equation For Hydrogen Fuel Cells.
From www.chegg.com
Solved Suppose you have a hydrogenoxygen fuel cell with the Half Equation For Hydrogen Fuel Cells We can break down this overall reaction into two half. The (chemical) energy content of any fuel is. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. Half Equation For Hydrogen Fuel Cells.
From www.slideserve.com
PPT From Voltage Cells to Nernst Equation PowerPoint Presentation Half Equation For Hydrogen Fuel Cells The (chemical) energy content of any fuel is. It has a cell potential of 0.00v, measured. a somewhat oversimplified diagram of a fuel cell in which the cell reaction is the production of water from hydrogen and oxygen is shown in figure. the overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water.. Half Equation For Hydrogen Fuel Cells.