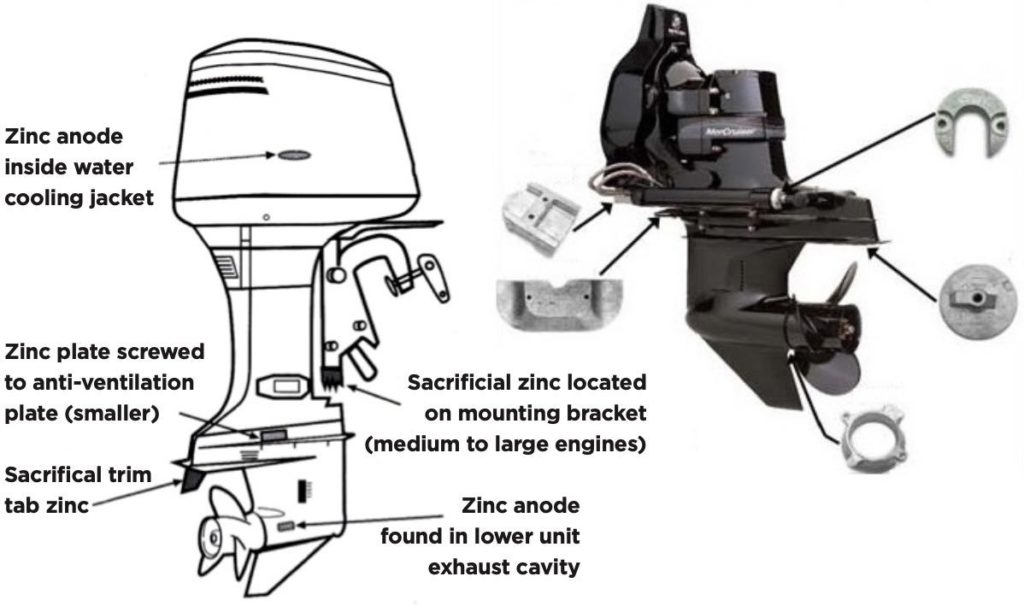
Why Do You Put Zinc On Boats . This deterioration of metals is what’s known as galvanic corrosion. When you immerse two metals with different nobilities in an electrolyte (such as. What is the purpose of a zinc anode on a boat? the basic premise of galvanic corrosion is this: there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. Zinc anodes are “sacrificial” attachments that protect submerged structures from. Zinc protects well in salt, fair. what are zinc anodes for on boats? To stop galvanic corrosion from destroying your underwater. zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. sacrificial anodes (zincs) stop galvanic corrosion. Let’s go over why you put zinc anodes on boats. anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals.
from islandfishermanmagazine.com
the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals. To stop galvanic corrosion from destroying your underwater. sacrificial anodes (zincs) stop galvanic corrosion. anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. What is the purpose of a zinc anode on a boat? the basic premise of galvanic corrosion is this: there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. what are zinc anodes for on boats? Zinc protects well in salt, fair. When you immerse two metals with different nobilities in an electrolyte (such as.
Is Your Boat Voltage Tuned For Fishing? Page 2 of 2 Island
Why Do You Put Zinc On Boats Zinc anodes are “sacrificial” attachments that protect submerged structures from. What is the purpose of a zinc anode on a boat? zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. Zinc anodes are “sacrificial” attachments that protect submerged structures from. the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals. When you immerse two metals with different nobilities in an electrolyte (such as. anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. This deterioration of metals is what’s known as galvanic corrosion. Zinc protects well in salt, fair. To stop galvanic corrosion from destroying your underwater. Let’s go over why you put zinc anodes on boats. the basic premise of galvanic corrosion is this: sacrificial anodes (zincs) stop galvanic corrosion. what are zinc anodes for on boats?
From www.jon-boats-for-sale.com
Best Jon Boat Float Pods. How to Choose Float Pods for Your Boat? Jon Why Do You Put Zinc On Boats the basic premise of galvanic corrosion is this: Zinc protects well in salt, fair. To stop galvanic corrosion from destroying your underwater. Let’s go over why you put zinc anodes on boats. there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. Zinc anodes are “sacrificial” attachments that protect submerged structures. Why Do You Put Zinc On Boats.
From www.lakewizard.com
What Is Outdrive On A Boat? LakeWizard Why Do You Put Zinc On Boats Zinc protects well in salt, fair. When you immerse two metals with different nobilities in an electrolyte (such as. there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. Zinc anodes are “sacrificial” attachments that protect submerged structures from. This deterioration of metals is what’s known as galvanic corrosion. what are. Why Do You Put Zinc On Boats.
From www.frontiersin.org
Frontiers Functions and strategies for enhancing zinc availability in Why Do You Put Zinc On Boats Zinc anodes are “sacrificial” attachments that protect submerged structures from. the basic premise of galvanic corrosion is this: sacrificial anodes (zincs) stop galvanic corrosion. What is the purpose of a zinc anode on a boat? zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. When you immerse two metals. Why Do You Put Zinc On Boats.
From ar.inspiredpencil.com
Roof Leaking What To Do Why Do You Put Zinc On Boats the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals. what are zinc anodes for on boats? What is the purpose of a zinc anode on a boat? When you immerse two metals with different nobilities in an electrolyte (such as. To. Why Do You Put Zinc On Boats.
From www.stepbystep.com
How to Position Zinc Plates on Boats Why Do You Put Zinc On Boats To stop galvanic corrosion from destroying your underwater. What is the purpose of a zinc anode on a boat? Zinc anodes are “sacrificial” attachments that protect submerged structures from. anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. sacrificial anodes (zincs) stop galvanic. Why Do You Put Zinc On Boats.
From wirelibrarybrahmins.z22.web.core.windows.net
Cathode Electrolyte Circuit Diagram Why Do You Put Zinc On Boats what are zinc anodes for on boats? anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. Zinc anodes are “sacrificial” attachments that protect submerged structures from. zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your. Why Do You Put Zinc On Boats.
From bdoutdoors.com
Why Zincs Are So Important On Boats Bloodydecks Why Do You Put Zinc On Boats there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. This deterioration of metals is what’s known as galvanic corrosion. the basic premise of galvanic corrosion is this: Zinc protects well in salt,. Why Do You Put Zinc On Boats.
From www.boatoutfitters.com
Aluminum vs. Zinc Anodes for Boats Saltwater and Freshwater Why Do You Put Zinc On Boats the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals. zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. When you immerse two metals with different nobilities in an electrolyte (such as. sacrificial. Why Do You Put Zinc On Boats.
From www.scyr.org
Zinc Locations Why Do You Put Zinc On Boats what are zinc anodes for on boats? anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. This deterioration of metals is what’s known as galvanic. Why Do You Put Zinc On Boats.
From ar.inspiredpencil.com
Please Do Not Use Sign Why Do You Put Zinc On Boats zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals. Zinc protects well in salt, fair. there are a few reasons why many manufacturers. Why Do You Put Zinc On Boats.
From www.desertcart.lk
Buy T A Paints LtdMarine Boat Barge Primer Undercoat Anti Corrosive Why Do You Put Zinc On Boats what are zinc anodes for on boats? zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. What is the purpose of a zinc anode on a boat? When you immerse two metals with different nobilities in an electrolyte (such as. anodes are typically made of zinc, which is an. Why Do You Put Zinc On Boats.
From citimarinestore.com
When to Use Zinc Anodes or Aluminum Anodes on Your Boat Why Do You Put Zinc On Boats there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals. To stop galvanic corrosion from destroying your underwater. sacrificial anodes (zincs) stop galvanic corrosion.. Why Do You Put Zinc On Boats.
From achievetampabay.org
How To Install Zinc Roof Strips Without Nails? Update New Why Do You Put Zinc On Boats To stop galvanic corrosion from destroying your underwater. What is the purpose of a zinc anode on a boat? Zinc protects well in salt, fair. sacrificial anodes (zincs) stop galvanic corrosion. the basic premise of galvanic corrosion is this: When you immerse two metals with different nobilities in an electrolyte (such as. zinc anodes, also known as. Why Do You Put Zinc On Boats.
From www.youtube.com
JON BOAT FLOAT PODS GUIDE Everything you need to know! YouTube Why Do You Put Zinc On Boats zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. What is the purpose of a zinc anode on a boat? This deterioration of metals is what’s known as galvanic corrosion. anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic. Why Do You Put Zinc On Boats.
From www.alamy.com
Labelled diagram for laboratory preparation of hydrogen from zinc and Why Do You Put Zinc On Boats Let’s go over why you put zinc anodes on boats. To stop galvanic corrosion from destroying your underwater. sacrificial anodes (zincs) stop galvanic corrosion. This deterioration of metals is what’s known as galvanic corrosion. the basic premise of galvanic corrosion is this: there are a few reasons why many manufacturers and boaters use aluminum over zinc or. Why Do You Put Zinc On Boats.
From marineprotection.co.nz
Anode Specs Marine Protection Systems Why Do You Put Zinc On Boats the basic premise of galvanic corrosion is this: what are zinc anodes for on boats? When you immerse two metals with different nobilities in an electrolyte (such as. This deterioration of metals is what’s known as galvanic corrosion. sacrificial anodes (zincs) stop galvanic corrosion. To stop galvanic corrosion from destroying your underwater. Zinc protects well in salt,. Why Do You Put Zinc On Boats.
From www.myboatlife.com
Avoiding MerCruiser Bravo 3 Outdrive Corrosion Problems on Boats My Why Do You Put Zinc On Boats zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. the basic premise of galvanic corrosion is this: Let’s go over why you put zinc anodes on boats. what are zinc anodes for on boats? the reason zinc has to give up pieces of itself is because electrons are. Why Do You Put Zinc On Boats.
From www.silverstreakboats.com
16' Jet Boat Ultimate River Boat Aluminum Boat by Silver Streak Boats Why Do You Put Zinc On Boats To stop galvanic corrosion from destroying your underwater. what are zinc anodes for on boats? Zinc protects well in salt, fair. When you immerse two metals with different nobilities in an electrolyte (such as. the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the. Why Do You Put Zinc On Boats.
From www.britannica.com
zinc Properties, Uses, & Facts Britannica Why Do You Put Zinc On Boats zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. the basic premise of galvanic corrosion is this: Let’s go over why you put zinc anodes on boats. What is the purpose of a zinc anode on a boat? To stop galvanic corrosion from destroying your underwater. When you immerse two. Why Do You Put Zinc On Boats.
From blog.ultimatelifespan.com
Zinc The Famous Trace Mineral & Why You Need It Ultimate Lifespan Why Do You Put Zinc On Boats anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. When you immerse two metals with different nobilities in an electrolyte (such as. This deterioration of metals is what’s known as galvanic corrosion. zinc anodes, also known as sacrificial anodes, play a key role. Why Do You Put Zinc On Boats.
From blog.boats.com
Understanding Boat Zincs and Galvanic Corrosion Why Do You Put Zinc On Boats Zinc protects well in salt, fair. What is the purpose of a zinc anode on a boat? the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals. there are a few reasons why many manufacturers and boaters use aluminum over zinc or. Why Do You Put Zinc On Boats.
From www.slideserve.com
PPT The Importance of Zinc Anodes in Boats PowerPoint Presentation Why Do You Put Zinc On Boats zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. Zinc anodes are “sacrificial” attachments that protect submerged structures from. anodes are typically made of zinc, which is an extremely effective base metal. Why Do You Put Zinc On Boats.
From www.youtube.com
Guide to Replacing Zincs / Anodes on Your Boat zinc anode YouTube Why Do You Put Zinc On Boats Zinc anodes are “sacrificial” attachments that protect submerged structures from. anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. When you immerse two metals with different. Why Do You Put Zinc On Boats.
From www.propellerdepot.com
Why Zinc Anodes? Why Do You Put Zinc On Boats What is the purpose of a zinc anode on a boat? there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. what are zinc anodes for on boats? To stop galvanic corrosion from. Why Do You Put Zinc On Boats.
From www.youtube.com
single displacement reaction of Copper from Copper Sulphate Solution by Why Do You Put Zinc On Boats To stop galvanic corrosion from destroying your underwater. Let’s go over why you put zinc anodes on boats. What is the purpose of a zinc anode on a boat? Zinc protects well in salt, fair. what are zinc anodes for on boats? sacrificial anodes (zincs) stop galvanic corrosion. When you immerse two metals with different nobilities in an. Why Do You Put Zinc On Boats.
From www.youtube.com
Boat Zinc Why it Works {anodes} YouTube Why Do You Put Zinc On Boats When you immerse two metals with different nobilities in an electrolyte (such as. zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. the basic premise of galvanic corrosion is this: what are zinc anodes for on boats? This deterioration of metals is what’s known as galvanic corrosion. there. Why Do You Put Zinc On Boats.
From australianwoodenboatfestival.com.au
Marine Corrosion Are zinc anodes OK on wooden boats? Australian Why Do You Put Zinc On Boats anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. Zinc anodes are “sacrificial” attachments that protect submerged structures from. Let’s go over why you put zinc anodes on boats. there are a few reasons why many manufacturers and boaters use aluminum over zinc. Why Do You Put Zinc On Boats.
From www.youtube.com
SPRING BOAT PREP How to Replace Zinc Anodes to Prevent Corrosion on Why Do You Put Zinc On Boats This deterioration of metals is what’s known as galvanic corrosion. Zinc protects well in salt, fair. Zinc anodes are “sacrificial” attachments that protect submerged structures from. To stop galvanic corrosion from destroying your underwater. sacrificial anodes (zincs) stop galvanic corrosion. the reason zinc has to give up pieces of itself is because electrons are created as the metal. Why Do You Put Zinc On Boats.
From www.myboatlife.com
How to Replace Sacrificial Zinc Anodes on Your Boat My Boat Life Why Do You Put Zinc On Boats anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. sacrificial anodes (zincs) stop galvanic corrosion. the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals. . Why Do You Put Zinc On Boats.
From www.youtube.com
Beavertail Aluminum Boat Floatation Pods Weldon Before and After Why Do You Put Zinc On Boats anodes are typically made of zinc, which is an extremely effective base metal far less resistant to galvanic and electrolytic corrosion than most other. This deterioration of metals is what’s known as galvanic corrosion. When you immerse two metals with different nobilities in an electrolyte (such as. there are a few reasons why many manufacturers and boaters use. Why Do You Put Zinc On Boats.
From www.europeanmarinesurveys.com
Marine AnodesMarine SurveysEuropean Marine Services European Marine Why Do You Put Zinc On Boats To stop galvanic corrosion from destroying your underwater. When you immerse two metals with different nobilities in an electrolyte (such as. there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. Zinc protects well in salt, fair. the basic premise of galvanic corrosion is this: anodes are typically made of. Why Do You Put Zinc On Boats.
From www.boats.com
Understanding Boat Zincs and Galvanic Corrosion Why Do You Put Zinc On Boats what are zinc anodes for on boats? What is the purpose of a zinc anode on a boat? sacrificial anodes (zincs) stop galvanic corrosion. the reason zinc has to give up pieces of itself is because electrons are created as the metal dissolves, generating the current between the two metals. Zinc anodes are “sacrificial” attachments that protect. Why Do You Put Zinc On Boats.
From islandfishermanmagazine.com
Is Your Boat Voltage Tuned For Fishing? Page 2 of 2 Island Why Do You Put Zinc On Boats To stop galvanic corrosion from destroying your underwater. there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. Zinc protects well in salt, fair. zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. the basic premise of galvanic corrosion is this: Let’s. Why Do You Put Zinc On Boats.
From alternativemedicine.com
Why is Zinc so Important? Why Do You Put Zinc On Boats the basic premise of galvanic corrosion is this: This deterioration of metals is what’s known as galvanic corrosion. What is the purpose of a zinc anode on a boat? there are a few reasons why many manufacturers and boaters use aluminum over zinc or magnesium anodes. Zinc protects well in salt, fair. Let’s go over why you put. Why Do You Put Zinc On Boats.
From www.youtube.com
BARBARIC "BIG LICK" Canter Gait Championship Class Asheville "Big Why Do You Put Zinc On Boats what are zinc anodes for on boats? zinc anodes, also known as sacrificial anodes, play a key role in the maintenance of your boat. Let’s go over why you put zinc anodes on boats. sacrificial anodes (zincs) stop galvanic corrosion. Zinc protects well in salt, fair. anodes are typically made of zinc, which is an extremely. Why Do You Put Zinc On Boats.