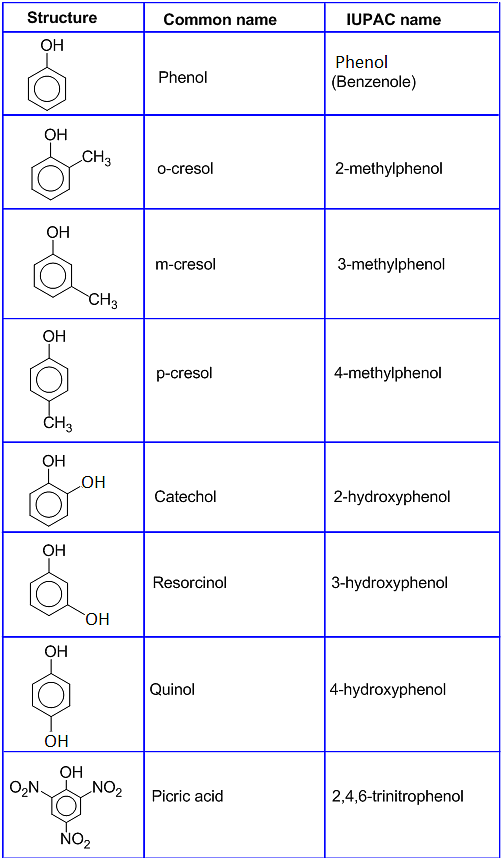
Alcohol And Phenols . Phenol is relatively strong an acid compared to. Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Know the name and uses of simple alcohols, phenols and ethers. Most alcohols are very weak acids. Properties of alcohols and phenols. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases.
from collegedunia.com
Properties of alcohols and phenols. Phenol is relatively strong an acid compared to. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Most alcohols are very weak acids. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom.
Nomenclature of Alcohols Phenols and Ethers Rules and Examples
Alcohol And Phenols Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Phenol is relatively strong an acid compared to. Know the name and uses of simple alcohols, phenols and ethers. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Most alcohols are very weak acids. Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. Properties of alcohols and phenols.
From chem.libretexts.org
17.S Alcohols and Phenols (Summary) Chemistry LibreTexts Alcohol And Phenols Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. Most alcohols are very weak acids. Properties of alcohols and phenols. An alcohol is an organic compound with. Alcohol And Phenols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Alcohol And Phenols Alcohols and phenols have nearly the same geometry around the oxygen atom as water. The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Phenol is relatively strong an acid compared to. Properties of alcohols and phenols. An alcohol is an organic compound with a hydroxyl (oh). Alcohol And Phenols.
From www.differencebetween.com
Difference Between Alcohols and Phenols Compare the Difference Alcohol And Phenols Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Explain. Alcohol And Phenols.
From thecontentauthority.com
Alcohol vs Phenol Meaning And Differences Alcohol And Phenols Phenol is relatively strong an acid compared to. Most alcohols are very weak acids. Properties of alcohols and phenols. Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Explain why the boiling points of alcohols and phenols. Alcohol And Phenols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Alcohol And Phenols Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Though the ph of an alcohol solution is almost neutral, they are still able. Alcohol And Phenols.
From www.cleariitmedical.com
Alcohols, Phenols and Ethers Chemistry Notes for IITJEE/NEET Alcohol And Phenols Properties of alcohols and phenols. Phenol is relatively strong an acid compared to. Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. An alcohol is an organic compound. Alcohol And Phenols.
From www.geeksforgeeks.org
Chemical reactions of Alcohols, Phenols and Ethers Alcohol And Phenols Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and. Alcohol And Phenols.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation ID233852 Alcohol And Phenols Know the name and uses of simple alcohols, phenols and ethers. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Phenol is relatively strong an acid compared to. Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Properties of alcohols and phenols. Explain. Alcohol And Phenols.
From pediaa.com
Difference Between Alcohol and Phenol Definition, Structure, Use Alcohol And Phenols Most alcohols are very weak acids. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Phenol is relatively strong an acid compared to. Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Know the name and uses of. Alcohol And Phenols.
From studylib.net
Alcohols, Phenols and Ethers Alcohol And Phenols Properties of alcohols and phenols. Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Know the name and uses of simple alcohols, phenols and ethers. Most alcohols are very weak acids. Phenol is relatively strong an acid compared to. An alcohol is an organic compound with a hydroxyl (oh) functional group. Alcohol And Phenols.
From chem.libretexts.org
17.S Alcohols and Phenols (Summary) Chemistry LibreTexts Alcohol And Phenols Most alcohols are very weak acids. Know the name and uses of simple alcohols, phenols and ethers. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Infrared, nuclear magnetic. Alcohol And Phenols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Alcohol And Phenols Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Most alcohols are very weak acids. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Phenol. Alcohol And Phenols.
From pediaa.com
Difference Between Alcohol and Phenol Definition, Structure, Use Alcohol And Phenols The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Know the name and uses of simple alcohols, phenols and ethers. Phenol is relatively strong an acid compared to. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Infrared, nuclear magnetic resonance. Alcohol And Phenols.
From www.youtube.com
Alcohol Phenols And Ether Class 12 Nomenclature Of Alcohol NCERT Alcohol And Phenols Know the name and uses of simple alcohols, phenols and ethers. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Most alcohols are very weak acids. Properties of alcohols and phenols. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular. Alcohol And Phenols.
From www.pw.live
Difference Between Alcohol And Phenol Alcohol And Phenols Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Know the name and uses of simple alcohols, phenols and ethers. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Though the ph of an alcohol solution is almost neutral, they are still able to react with. Alcohol And Phenols.
From www.youtube.com
Alcohol and Phenol YouTube Alcohol And Phenols Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic. Alcohol And Phenols.
From www.youtube.com
Difference Between Alcohol And Phenol?Class Series YouTube Alcohol And Phenols Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Alcohols and phenols have nearly the same geometry around the oxygen atom as water. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. The alcohols are a class of. Alcohol And Phenols.
From www.youtube.com
Chapter 17 Alcohols and Phenols / Lecture 2 (slides + voice record Alcohol And Phenols Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Phenol is relatively strong an acid compared to. Know. Alcohol And Phenols.
From www.geeksforgeeks.org
Nomenclature of Alcohols, Phenols and Ethers Rules and Examples Alcohol And Phenols Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Know the name and uses of simple alcohols, phenols and ethers. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Properties of alcohols and phenols. An alcohol is an organic compound with. Alcohol And Phenols.
From www.slideserve.com
PPT Chapter 14 Alcohols, Phenols, Ethers, and Thiols PowerPoint Alcohol And Phenols Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. Phenol is relatively strong an acid compared to. Most alcohols are very weak acids. Properties of alcohols and phenols. Know the name and uses of simple alcohols, phenols and ethers. The alcohols are a class of organic compounds that hold at. Alcohol And Phenols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Alcohol And Phenols The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Most alcohols are very weak acids. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Phenol is relatively strong an acid compared to.. Alcohol And Phenols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Alcohol And Phenols Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Know the name and uses of simple alcohols, phenols and ethers. Phenols, on the other hand, are organic compounds. Alcohol And Phenols.
From www.slideserve.com
PPT PHENOLS PowerPoint Presentation, free download ID9445745 Alcohol And Phenols Most alcohols are very weak acids. Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Phenol is relatively strong an acid compared to. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which. Alcohol And Phenols.
From www.imperialstudy.com
Alcohols Phenols and Ethers Notes for Class 12 Chemistry Imperial Study Alcohol And Phenols An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar. Alcohol And Phenols.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and Alcohol And Phenols Know the name and uses of simple alcohols, phenols and ethers. Phenol is relatively strong an acid compared to. Most alcohols are very weak acids. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is. Alcohol And Phenols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Alcohol And Phenols Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Infrared,. Alcohol And Phenols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Alcohol And Phenols An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. Infrared,. Alcohol And Phenols.
From collegedunia.com
Nomenclature of Alcohols Phenols and Ethers Rules and Examples Alcohol And Phenols Most alcohols are very weak acids. The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. Properties of alcohols and phenols. An alcohol is an organic compound with. Alcohol And Phenols.
From chem.libretexts.org
9.2 Alcohols and Phenols Nomenclature and Classification Chemistry Alcohol And Phenols Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about alcohols and phenols, and we. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of. Alcohol And Phenols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Alcohol And Phenols Properties of alcohols and phenols. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached. Alcohol And Phenols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Alcohol And Phenols Properties of alcohols and phenols. Phenol is relatively strong an acid compared to. Phenols, on the other hand, are organic compounds consisting of a hydroxyl group which is attached to an aromatic system of hydrocarbons (arene). Know the name and uses of simple alcohols, phenols and ethers. Though the ph of an alcohol solution is almost neutral, they are still. Alcohol And Phenols.
From chem.libretexts.org
17.S Alcohols and Phenols (Summary) Chemistry LibreTexts Alcohol And Phenols The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Know the name and uses of simple alcohols, phenols and ethers. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Properties of alcohols and. Alcohol And Phenols.
From www.slideshare.net
Alcohols, Phenols, and Ethers Alcohol And Phenols Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Phenol is relatively strong an acid compared to. An alcohol is an organic compound with a hydroxyl (oh) functional group on an aliphatic carbon atom. Alcohols and phenols have nearly the same geometry around the oxygen atom as. Alcohol And Phenols.
From studylib.net
Alcohols and Phenols Alcohol And Phenols The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Properties of alcohols and phenols. Know the name and uses of simple alcohols, phenols and ethers. Though the ph of an alcohol solution. Alcohol And Phenols.
From chem.libretexts.org
17.S Alcohols and Phenols (Summary) Chemistry LibreTexts Alcohol And Phenols The alcohols are a class of organic compounds that hold at least one hydroxyl functional group that is attached to a carbon atom. Though the ph of an alcohol solution is almost neutral, they are still able to react with strong bases. Properties of alcohols and phenols. Infrared, nuclear magnetic resonance and mass spectroscopy each can provide valuable information about. Alcohol And Phenols.