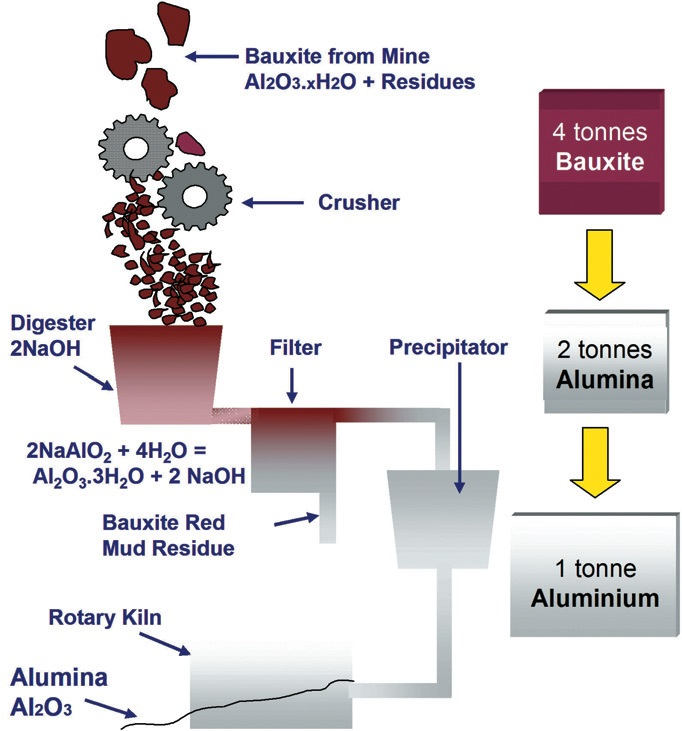
Bauxite To Aluminum . It results from the weathering,. The bauxite is purified by the bayer. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. Bauxite ore is the world’s primary source of aluminum. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. Refining bauxite to obtain alumina and smelting alumina to produce aluminum. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Bauxite contains a number of impurities, including iron oxide, silica, and titania. The ore must first be chemically processed to produce alumina (aluminum oxide).
from pdfprof.com
Bauxite ore is the world’s primary source of aluminum. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Refining bauxite to obtain alumina and smelting alumina to produce aluminum. The ore must first be chemically processed to produce alumina (aluminum oxide). The bauxite is purified by the bayer. It results from the weathering,. Bauxite contains a number of impurities, including iron oxide, silica, and titania. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates.
extraction de l'alumine de la bauxite
Bauxite To Aluminum The ore must first be chemically processed to produce alumina (aluminum oxide). It results from the weathering,. The ore must first be chemically processed to produce alumina (aluminum oxide). Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Bauxite contains a number of impurities, including iron oxide, silica, and titania. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Bauxite ore is the world’s primary source of aluminum. The bauxite is purified by the bayer. Refining bauxite to obtain alumina and smelting alumina to produce aluminum.
From www.fossilera.com
7.7" Polished Bauxite (Aluminum Ore) Slab Australia For Sale (46710 Bauxite To Aluminum Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. 4 tons of bauxite are needed to obtain 2 tons of alumina, from. Bauxite To Aluminum.
From mineralseducationcoalition.org
Aluminum Minerals Education Coalition Bauxite To Aluminum Refining bauxite to obtain alumina and smelting alumina to produce aluminum. It results from the weathering,. Bauxite ore is the world’s primary source of aluminum. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Bauxite is typically a mixture of hydroxides of aluminium with iron. Bauxite To Aluminum.
From www.sciencephoto.com
Bauxite, Aluminum ore, from Le Baux, France Stock Image C041/0600 Bauxite To Aluminum Refining bauxite to obtain alumina and smelting alumina to produce aluminum. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. The ore must first be chemically processed to produce alumina (aluminum. Bauxite To Aluminum.
From www.slideserve.com
PPT Options(electrochemistry) PowerPoint Presentation, free download Bauxite To Aluminum Refining bauxite to obtain alumina and smelting alumina to produce aluminum. Bauxite ore is the world’s primary source of aluminum. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. 4 tons. Bauxite To Aluminum.
From www.fossilera.com
6.3" Polished Bauxite (Aluminum Ore) Section Australia (221463) For Bauxite To Aluminum As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Bauxite contains a number of impurities, including iron oxide, silica, and titania. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. It results. Bauxite To Aluminum.
From npobjects.wordpress.com
Aluminum Production from bauxite mining to chemical processing The Bauxite To Aluminum As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. It results from the weathering,. Refining bauxite to obtain alumina and smelting alumina to. Bauxite To Aluminum.
From pdfprof.com
extraction de l'alumine de la bauxite Bauxite To Aluminum 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. The ore must first be chemically processed to produce alumina (aluminum oxide). Bauxite contains a number of impurities, including iron oxide, silica, and titania. The bauxite is purified by the bayer. As the minerals are weathered they gradually. Bauxite To Aluminum.
From www.ftmmachinery.com
Bauxite Beneficiation Efficient Way to Remove Silica in Bauxite Fote Bauxite To Aluminum The ore must first be chemically processed to produce alumina (aluminum oxide). Bauxite ore is the world’s primary source of aluminum. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. Refining bauxite to obtain alumina and smelting alumina to produce aluminum. It results from the weathering,. The. Bauxite To Aluminum.
From www.researchgate.net
Bayer process for the production of alumina from bauxite. Download Bauxite To Aluminum The ore must first be chemically processed to produce alumina (aluminum oxide). As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Primary production involves mining bauxite deposits from the earth,. Bauxite To Aluminum.
From www.oresomeresources.com
Bauxite Oresome Resources Bauxite To Aluminum 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. It results from the weathering,. Refining bauxite to obtain alumina and smelting alumina to produce aluminum. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Primary production involves mining bauxite deposits from. Bauxite To Aluminum.
From blog.thepipingmart.com
Extraction of Aluminium Step By Step Guide Bauxite To Aluminum Bauxite ore is the world’s primary source of aluminum. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. It results from the weathering,. As. Bauxite To Aluminum.
From www.basystems.co.uk
The Manufacturing Basics of Aluminium BA Systems Bauxite To Aluminum Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. The ore must first be chemically processed to produce alumina (aluminum oxide). Bauxite contains a number of impurities, including iron oxide, silica, and titania. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to. Bauxite To Aluminum.
From www.sandatlas.org
Bauxite Bauxite To Aluminum It results from the weathering,. Bauxite contains a number of impurities, including iron oxide, silica, and titania. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. The bauxite is purified by the bayer. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum. Bauxite To Aluminum.
From www.researchgate.net
2 Extraction route of aluminium from the initial bauxite step to the Bauxite To Aluminum Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Bauxite ore is the world’s primary source of aluminum. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. The bauxite is purified by the bayer. 4 tons of bauxite are needed. Bauxite To Aluminum.
From www.qurails.com
Production Process • Qurails Bauxite To Aluminum Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Bauxite contains a number of impurities, including iron oxide, silica, and titania. The bauxite is purified by the bayer. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Primary production. Bauxite To Aluminum.
From www.alamy.com
Karst type bauxite (ore of aluminum Stock Photo Alamy Bauxite To Aluminum The ore must first be chemically processed to produce alumina (aluminum oxide). The bauxite is purified by the bayer. It results from the weathering,. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o,. Bauxite To Aluminum.
From www.researchgate.net
Components of bauxite consist of aluminum oxide that is dissolved Bauxite To Aluminum Bauxite ore is the world’s primary source of aluminum. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. The bauxite is purified by the bayer. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. The ore must first be. Bauxite To Aluminum.
From www.sciencephoto.com
Bauxite and aluminium Stock Image C026/6671 Science Photo Library Bauxite To Aluminum Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. It results from the weathering,. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. Bauxite contains a number of impurities, including iron oxide, silica, and titania. 4 tons of bauxite are. Bauxite To Aluminum.
From www.youtube.com
Extraction of Aluminium from Bauxite YouTube Bauxite To Aluminum Refining bauxite to obtain alumina and smelting alumina to produce aluminum. It results from the weathering,. The ore must first be chemically processed to produce alumina (aluminum oxide). Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and. Bauxite To Aluminum.
From www.youtube.com
Extraction of Aluminium from Bauxite ICSE Class 10 Bayer’s Hall Bauxite To Aluminum Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known. Bauxite To Aluminum.
From www.alamy.com
Bauxite, Brazil, aluminum ore, sedimentary rock , aluminum oxides Stock Bauxite To Aluminum It results from the weathering,. Bauxite ore is the world’s primary source of aluminum. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Bauxite contains a number of impurities, including iron oxide, silica, and titania. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is. Bauxite To Aluminum.
From www.fossilera.com
4.1" Polished Bauxite (Aluminum Ore) Slab Australia (46685) For Sale Bauxite To Aluminum Bauxite ore is the world’s primary source of aluminum. It results from the weathering,. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. The bauxite is purified by the bayer. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum. Bauxite To Aluminum.
From differencebetweenz.com
Difference between Bauxite and Aluminum Difference Betweenz Bauxite To Aluminum Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. Bauxite contains a number of impurities, including iron oxide, silica, and titania. The bauxite is. Bauxite To Aluminum.
From www.fossilera.com
3.4" Polished Bauxite (Aluminum Ore) Slab Australia For Sale (46669 Bauxite To Aluminum 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form.. Bauxite To Aluminum.
From geologypics.com
Bauxite aluminum ore Geology Pics Bauxite To Aluminum Refining bauxite to obtain alumina and smelting alumina to produce aluminum. Bauxite contains a number of impurities, including iron oxide, silica, and titania. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum. Bauxite To Aluminum.
From www.alamy.com
bauxite ore and aluminum stone together on isolated white background Bauxite To Aluminum As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Bauxite contains a number of impurities, including iron oxide, silica, and titania. The bauxite is purified by the bayer. The ore must first be chemically processed to produce alumina (aluminum oxide). Primary production involves mining bauxite. Bauxite To Aluminum.
From pixels.com
Bauxite And Aluminum Processing Complex Photograph by Randolph King Bauxite To Aluminum The bauxite is purified by the bayer. Bauxite ore is the world’s primary source of aluminum. Bauxite contains a number of impurities, including iron oxide, silica, and titania. Refining bauxite to obtain alumina and smelting alumina to produce aluminum. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Primary production involves mining bauxite deposits. Bauxite To Aluminum.
From www.youtube.com
Producing Endless Possibilities Alcoa Global Bauxite YouTube Bauxite To Aluminum Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. 4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis. The bauxite is purified by the bayer. Bauxite is typically a mixture of hydroxides of. Bauxite To Aluminum.
From www.dreamstime.com
Bauxite Ore and Aluminum Stone Together on Isolated White Background Bauxite To Aluminum Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form. Bauxite contains a number of impurities, including iron oxide, silica, and titania. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Refining. Bauxite To Aluminum.
From www.histalu.org
Histalu Aluminium Production From Bauxite to Alumina Bauxite To Aluminum Bauxite ore is the world’s primary source of aluminum. Refining bauxite to obtain alumina and smelting alumina to produce aluminum. It results from the weathering,. Bauxite contains a number of impurities, including iron oxide, silica, and titania. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to ultimately form.. Bauxite To Aluminum.
From www.researchgate.net
Blockflow diagram of primary aluminium and bauxite residue production Bauxite To Aluminum Bauxite ore is the world’s primary source of aluminum. It results from the weathering,. The ore must first be chemically processed to produce alumina (aluminum oxide). As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. 4 tons of bauxite are needed to obtain 2 tons. Bauxite To Aluminum.
From www.slideserve.com
PPT Extraction of metals PowerPoint Presentation ID208800 Bauxite To Aluminum Refining bauxite to obtain alumina and smelting alumina to produce aluminum. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. It results from the weathering,. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing. Bauxite To Aluminum.
From www.sciencephoto.com
Aluminium and bauxite Stock Image C044/0253 Science Photo Library Bauxite To Aluminum As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Primary production involves mining bauxite deposits from the earth, chemically refining it into pure aluminum oxide and performing electrometallurgical processing to. Bauxite To Aluminum.
From www.ftmmachinery.com
How to Get Aluminum from Bauxite Reduction Process Fote Machinery Bauxite To Aluminum The ore must first be chemically processed to produce alumina (aluminum oxide). Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. Refining bauxite to obtain alumina and smelting alumina to produce aluminum. The bauxite is purified by the bayer. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide,. Bauxite To Aluminum.
From www.fossilera.com
3.6" Polished Bauxite (Aluminum Ore) Slab Australia (46690) For Sale Bauxite To Aluminum Bauxite is typically a mixture of hydroxides of aluminium with iron oxides, silica and aluminosilicates. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, al 2 o 3.xh 2 o, known as bauxite. Bauxite contains a number of impurities, including iron oxide, silica, and titania. Refining bauxite to obtain alumina and smelting alumina to. Bauxite To Aluminum.