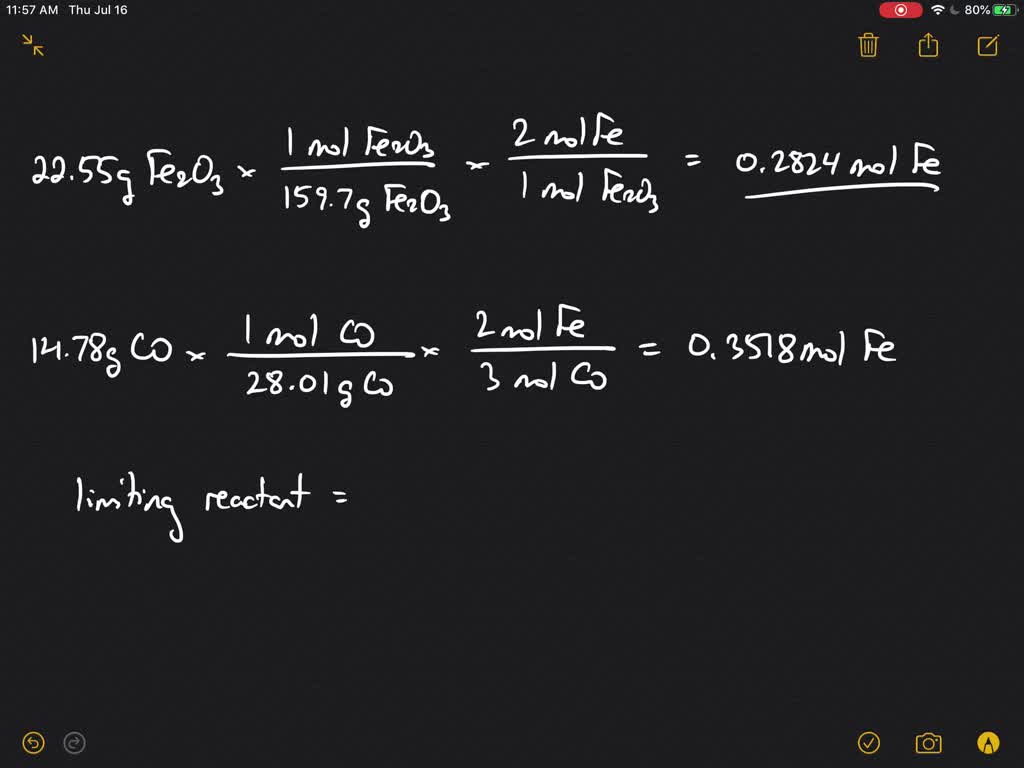
Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation . Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: Lioh + cro3 = crli2o4 + h2o. Write balanced equation for the reaction: If the reaction of 65.0. Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron metal and carbon dioxide by the reaction: Iron (iii) oxide reacts with carbon monoxide according to the equation: According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Fe2o3 (s) + 3co (g) to 2fe (s) +. Use the balanced chemical equation to find the stoichiometric ratios. To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Na + br2ca = ca + brna. Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g).
from www.numerade.com
Lioh + cro3 = crli2o4 + h2o. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Use the balanced chemical equation to find the stoichiometric ratios. Fe2o3 (s) + 3co (g) to 2fe (s) +. If the reaction of 65.0. According to the equation, 1 mole of fe2o3 reacts with 3 moles of co.
SOLVEDIron(III) oxide reacts with carbon monoxide according to the
Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Fe2o3 (s) + 3co (g) to 2fe (s) +. According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Na + br2ca = ca + brna. To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Write balanced equation for the reaction: Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Iron (iii) oxide reacts with carbon monoxide according to the equation: If the reaction of 65.0. Use the balanced chemical equation to find the stoichiometric ratios. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron metal and carbon dioxide by the reaction: Fe2o3 (s) + 3co (g) to 2fe (s) +. Lioh + cro3 = crli2o4 + h2o. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation:
From www.youtube.com
when iron rusts, solid iron reacts with gaseous oxygen to form solid Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. If the reaction of 65.0. Na + br2ca = ca + brna. Fe2o3 (s) + 3co (g) to 2fe (s) +. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: Iron (iii) oxide reacts with. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
Iron(III) oxide reacts with carbon monoxide according to the equation Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Iron (iii) oxide reacts with carbon monoxide according to the equation: Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Lioh + cro3 = crli2o4 + h2o. According to the equation, 1 mole of fe2o3 reacts. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.slideserve.com
PPT What is the difference between a chemical reaction and physical Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation If the reaction of 65.0. Use the balanced chemical equation to find the stoichiometric ratios. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Na + br2ca = ca + brna. To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Lioh + cro3 = crli2o4 + h2o. Iron (iii) oxide reacts. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVED Question 2 10 pts In a redox reaction, solid iron (III) oxide Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Write balanced equation for the reaction: Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.slideserve.com
PPT STOICHIOMETRY PowerPoint Presentation, free download ID3997530 Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Lioh + cro3 = crli2o4 + h2o. To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Iron (iii) oxide reacts with carbon monoxide according to the equation: Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. If the reaction of 65.0. Write balanced equation for. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVED47. Iron(III) oxide reacts with carbon monoxide equation Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation If the reaction of 65.0. Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron metal and carbon dioxide by the reaction: According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Iron(iii) oxide. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.slideserve.com
PPT STOICHIOMETRY PowerPoint Presentation, free download ID3997530 Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: Iron (iii) oxide reacts with. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.youtube.com
Iron (III) oxide on heating with carbon monoxide gas reacts to form Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. If the reaction of 65.0. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Iron (iii) oxide reacts with carbon monoxide according to the. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.vrogue.co
What Is Formed In The Reaction Between Iron And Oxyge vrogue.co Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron metal and carbon dioxide by the reaction: Na + br2ca = ca + brna. According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Iron (iii) oxide reacts with. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.coursehero.com
[Solved] Iron (III) oxide reacts with carbon monoxide to produce Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Use the balanced chemical equation to find the stoichiometric ratios. Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Iron (iii) oxide reacts with carbon monoxide. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.studypool.com
SOLUTION Iron(III) oxide reacts with carbon monoxide according to the Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Na + br2ca = ca + brna. Fe2o3 (s) + 3co (g) to 2fe (s) +. Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. If the reaction of 65.0. Iron (iii) oxide (fe2o3). Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
Iron(III) oxide reacts with carbon monoxide according to the equation Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Use the balanced chemical equation to find the stoichiometric ratios. To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Fe2o3 (s) + 3co (g) to 2fe (s) +. Cu (no3)2 + kno3 = cuno3 + k (no3)2. According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Write. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVED Iron (III) oxide reacts with carbon monoxide to produce iron Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Use the balanced chemical equation to find the stoichiometric ratios. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: Lioh + cro3 = crli2o4 + h2o. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron metal and carbon dioxide by. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVEDIron(III) oxide reacts with carbon monoxide according to the Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Write balanced equation for the reaction: Fe2o3 (s) + 3co (g) to 2fe (s) +. Na + br2ca = ca + brna. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: Iron (iii) oxide reacts with carbon monoxide according to the equation: If the reaction of 65.0. Fe 2 o 3. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVEDIron(III) oxide reacts with carbon monoxide according to the Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.chegg.com
Solved Iron (II) Oxide Reacts With Carbon Monoxide To For... Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Write balanced equation for the reaction: Na + br2ca = ca + brna. According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Fe2o3 (s) + 3co (g) to 2fe (s) +. Iron (iii) oxide. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation If the reaction of 65.0. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Lioh + cro3 = crli2o4 + h2o. To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Iron(iii) oxide reacts with carbon. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVED Solid iron(III) oxide reacts with carbon monoxide gas to form Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Cu (no3)2 + kno3 = cuno3 + k (no3)2. Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Lioh + cro3 = crli2o4 + h2o. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: According to the equation, 1. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the reaction that occurs when (a Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Write balanced equation for the reaction: Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Solid iron(iii) oxide reacts with carbon monoxide gas to. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.answersarena.com
[Solved] Please refer to image attached Iron(III) oxide re Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron metal and carbon dioxide by the reaction: Iron (iii) oxide reacts with carbon monoxide according to the equation: According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Write balanced equation for the reaction: Lioh + cro3 = crli2o4 + h2o. Solid iron(iii) oxide reacts. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.slideserve.com
PPT Stoichiometry with a Twist PowerPoint Presentation ID1836706 Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Use the balanced chemical equation to find the stoichiometric ratios. Na + br2ca = ca + brna. Lioh + cro3 = crli2o4 + h2o. Fe2o3 (s) + 3co (g) to 2fe (s) +. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation:. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From signalticket9.pythonanywhere.com
Spectacular Iron Oxide State Symbol Www Physics Wallah Com Notes Pdf Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron metal and carbon dioxide by the reaction: Use the balanced chemical equation to find the stoichiometric ratios. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Cu (no3)2 + kno3 =. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.chegg.com
Solved Question 5 (4 points) Iron(III) oxide reacts with CO Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Fe2o3 (s) + 3co (g) to 2fe (s) +. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: Fe2o3 (s) + 3co (g) →2fe (s) +. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.toppr.com
The balanced chemical equation for the reaction of iron (III) oxide and Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Write balanced equation for the reaction: Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. If the reaction of 65.0. According to the equation, 1 mole of fe2o3 reacts with 3 moles of co.. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.slideshare.net
Chemical Reactions Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Lioh + cro3 = crli2o4 + h2o. If the reaction of 65.0. Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron metal and carbon dioxide by. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.coursehero.com
[Solved] Iron(III) oxide reacts with carbon monoxide to produce Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). If the reaction of 65.0. Lioh + cro3 = crli2o4 + h2o. Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Use the balanced chemical equation to find the stoichiometric ratios. Cu (no3)2 + kno3 = cuno3 + k (no3)2. According to the. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVED In a blast furnace, coke, which is solid carbon, reacts with O2 Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Fe2o3 (s) + 3co (g) to 2fe (s) +. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Use the balanced chemical equation to find the stoichiometric ratios. If the reaction of 65.0. Cu (no3)2 + kno3 =. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.chegg.com
Solved Exercise 4.47 Iron(III) oxide reacts with carbon Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Lioh + cro3 = crli2o4 + h2o. Fe2o3 (s) + 3co (g) to 2fe (s) +. Iron (iii) oxide (fe2o3) combines with carbon monoxide to form iron metal and carbon dioxide by the reaction: Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. To balance. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Use the balanced chemical equation to find the stoichiometric ratios. Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Na + br2ca = ca + brna. Iron (iii) oxide reacts with carbon monoxide according to the equation: To balance fe2o3 + co = fe + co2 you'll need. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.slideserve.com
PPT 29 When 84.8 g of iron (III) oxide reacts with excess of carbon Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Use the balanced chemical equation to find the stoichiometric ratios. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Fe2o3 (s) + 3co (g) to 2fe (s) +. Cu. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVEDIron(III) oxide reacts with carbon monoxide to give iron metal Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. Na + br2ca = ca + brna. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: Use the balanced chemical. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVED Other '19.In a process known as smelting; iron (Ill) oxide Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Use the balanced chemical equation to find the stoichiometric ratios. Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2 (g) a reaction mixture. To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. According to. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From www.numerade.com
SOLVEDThe multistep smelting of ferric oxide to form elemental iron Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Iron(iii) oxide reacts with carbon monoxide to produce iron and carbon dioxide according to the balanced chemical equation: Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Fe 2 o 3 (s) + 3 co (g) → 2 fe (s) + 3 co 2. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From kunduz.com
[ANSWERED] Iron (III) oxide reacts with carbon monoxide according to Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation Use the balanced chemical equation to find the stoichiometric ratios. Lioh + cro3 = crli2o4 + h2o. According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Write balanced equation for the reaction: Fe2o3 (s) + 3co (g) →2fe (s) + 3co2 (g). To balance fe2o3 + co = fe + co2 you'll need to watch. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.
From slideplayer.com
(D) OXIDISING AND REDUCING AGENTS ppt download Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation To balance fe2o3 + co = fe + co2 you'll need to watch out for two things. According to the equation, 1 mole of fe2o3 reacts with 3 moles of co. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Solid iron(iii) oxide reacts with carbon monoxide gas to produce solid iron and carbon dioxide gas. Write balanced equation for. Iron 3 Oxide Reacts With Carbon Monoxide Balanced Equation.