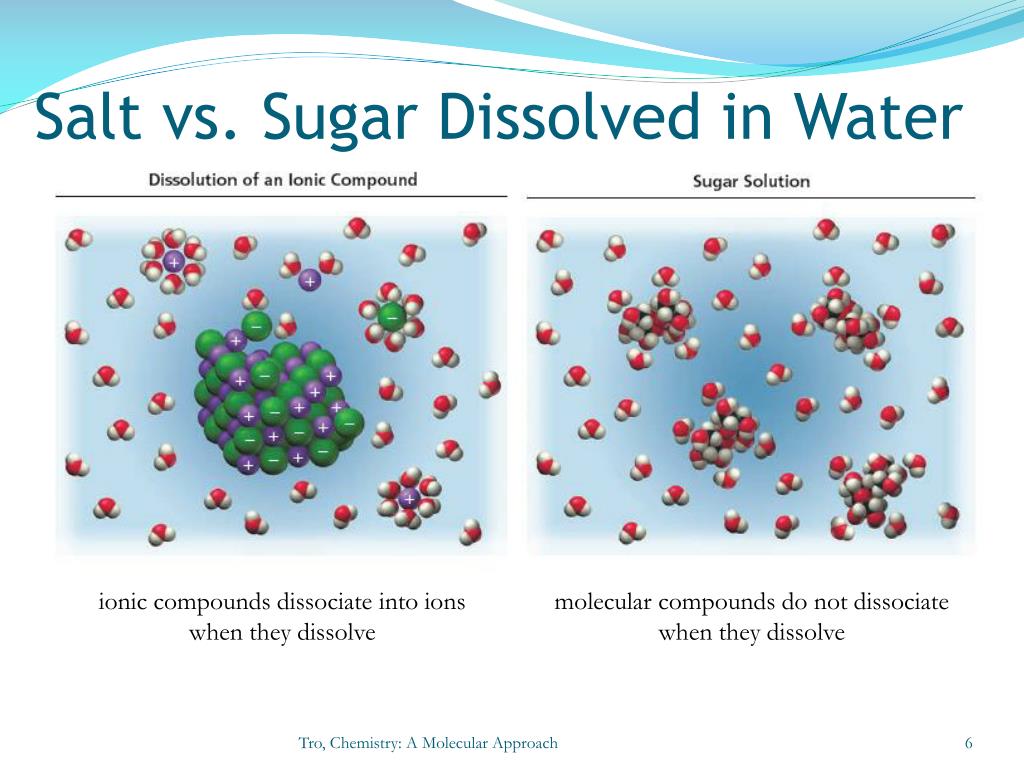
Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level . Water typically dissolves most ionic compounds and polar molecules. The most common way is to define the number of moles of solute per liter of solution. There are a number of ways to describe solubility. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. We will first examine the process that occurs when an ionic compound, such as table. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. Nonpolar molecules, such as those found in grease or oil,.
from www.slideserve.com
There are a number of ways to describe solubility. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. We will first examine the process that occurs when an ionic compound, such as table. Water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules, such as those found in grease or oil,. The most common way is to define the number of moles of solute per liter of solution. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between.
PPT Chapter 4 PowerPoint Presentation, free download ID762021
Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level The most common way is to define the number of moles of solute per liter of solution. We will first examine the process that occurs when an ionic compound, such as table. Water typically dissolves most ionic compounds and polar molecules. There are a number of ways to describe solubility. The most common way is to define the number of moles of solute per liter of solution. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. Nonpolar molecules, such as those found in grease or oil,. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Nonpolar molecules, such as those found in grease or oil,. We will first examine the process that occurs when an ionic compound, such as table. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water typically dissolves most ionic compounds and polar molecules. Water can dissolve salt because the positive part of water molecules. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. Nonpolar molecules, such as those. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.teachoo.com
Characterstics of Particles of Matter Class 9 Science Notes Teacho Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Water typically dissolves most ionic compounds and polar molecules. There are a number of ways to describe solubility. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. We will first examine the process that occurs when an ionic compound, such as table. Nonpolar molecules, such. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From favpng.com
Ionic Compound Sodium Chloride Ionic Bonding Chemical Compound, PNG Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level There are a number of ways to describe solubility. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. The most common way is to define the number of moles of solute per liter of solution. Water can dissolve salt. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From slideplayer.com
Water. ppt download Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. The most common way is to define the number of moles of solute per liter of solution. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.numerade.com
SOLVED You notice that NaCl salt easily dissolves in water, but does Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level We will first examine the process that occurs when an ionic compound, such as table. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water.. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From arianewsmcdowell.blogspot.com
Describe the Dissolving Process at the Molecular Level Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Nonpolar molecules, such as those found in grease or oil,. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. There are a number of ways to describe. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level The most common way is to define the number of moles of solute per liter of solution. We will first examine the process that occurs when an ionic compound, such as table. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. Nonpolar molecules, such. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.youtube.com
Why Salt (NaCl) Does Not Dissolve in Oil Salt and Oil Experiment Is Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. The most common way is to define the number of moles of solute per liter of solution. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From slideplayer.com
Basic units of EVERYTHING! ppt download Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level There are a number of ways to describe solubility. Nonpolar molecules, such as those found in grease or oil,. We will first examine the process that occurs when an ionic compound, such as table. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. The. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Nonpolar molecules, such as those found in grease or oil,. Water can dissolve salt because the positive part of water molecules. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From slideplayer.com
(solids) Solutions and Other Mixtures ppt download Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. There are a number of ways to describe solubility. Water typically. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Water typically dissolves most ionic compounds and polar molecules. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. There are a number of ways to describe solubility. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. Water typically. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. There are a number of ways to describe solubility. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From slideplayer.com
Ch. 13 Solutions What is a solution? ppt download Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The most common way is to define the number of moles of solute per liter of solution. Nonpolar molecules, such as those found in grease or oil,. Nonpolar molecules, such as those found. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.youtube.com
The Process of dissolving YouTube Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level We will first examine the process that occurs when an ionic compound, such as table. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. There are a number of ways to describe solubility. Water can dissolve salt because the positive part of water molecules. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. We will first examine the process that occurs when an ionic compound, such as table. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level There are a number of ways to describe solubility. Water typically dissolves most ionic compounds and polar molecules. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Nonpolar molecules,. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.slideshare.net
Solutions Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Nonpolar molecules, such as those found in grease or oil,. The most common way is to define the number of moles of solute per liter of solution. There are a number of ways. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From users.highland.edu
Aqueous Solutions Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Nonpolar molecules, such as those found in grease or oil,. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. Nonpolar molecules, such as those found. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From slideplayer.com
Properties of Compounds ppt download Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. Although much of the explanation for why certain substances. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From energyeducation.ca
Solute Energy Education Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Water typically dissolves most ionic compounds and polar molecules. At the molecular level, salt dissolves in water due. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level The most common way is to define the number of moles of solute per liter of solution. Nonpolar molecules, such as those found in grease or oil,. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Although much of. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2493612 Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.chegg.com
Solved Explain why NaCl does not dissolve in gasoline. Delta Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. There are a number of ways to describe solubility. We will first examine the process that occurs when an ionic compound, such as table. Nonpolar molecules, such as those found. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From courses.lumenlearning.com
The Dissolution Process Chemistry for Majors Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The most common way is to define the number. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From chem.libretexts.org
13.2 Solubility and Molecular Structure Chemistry LibreTexts Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Water typically dissolves most ionic compounds and polar molecules. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Nonpolar molecules, such as those found in grease or oil,. Very. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From brainly.in
the substance that will not dissolve in water Brainly.in Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level We will first examine the process that occurs when an ionic compound, such as table. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Water can dissolve salt because the positive part of water molecules attracts the negative chloride. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level The most common way is to define the number of moles of solute per liter of solution. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking of intermolecular forces between. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. We will first examine the process that occurs when an ionic compound, such as table.. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level We will first examine the process that occurs when an ionic compound, such as table. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water can dissolve salt because. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level We will first examine the process that occurs when an ionic compound, such as table. Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class,. Very simply, you explain the reason for this solubility rule by taking in consideration the energy requirements for the breaking. Salt Does Not Dissolve In Gasoline. Why Explain This At The Molecular Level.