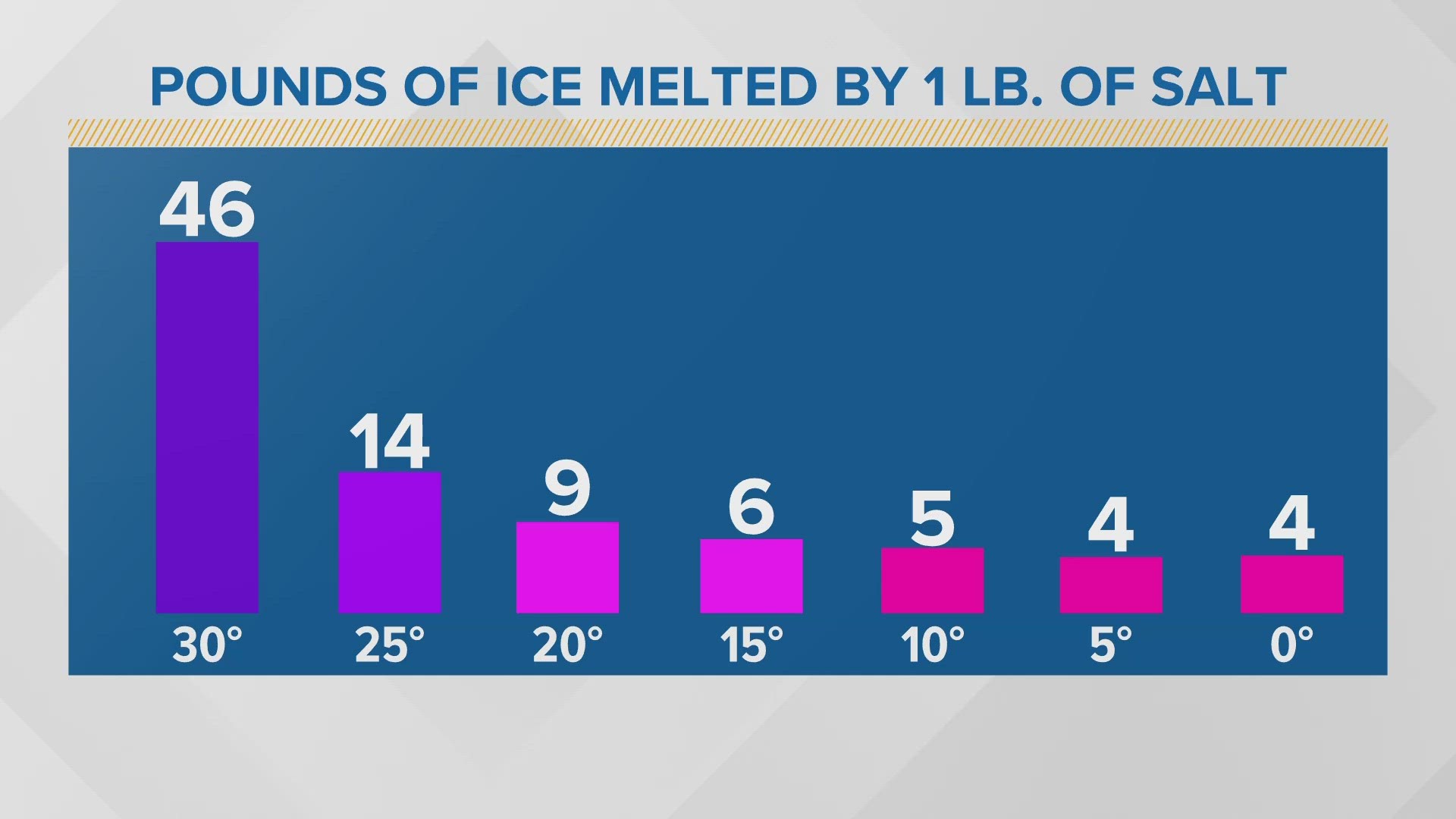
Salt Melt Ice Temperature . Salt lowers the water's freezing point via freezing point depression. Salt only helps if there is a little bit of liquid water available. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. At this temperature, your icy road generally has a thin layer of. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. The more surface area salt can cover, the better the chances for melting ice. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. This phenomenon is called freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. The salt has to dissolve into its ions in order to work.
from www.newscentermaine.com
When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The salt has to dissolve into its ions in order to work. At this temperature, your icy road generally has a thin layer of. Salt only helps if there is a little bit of liquid water available. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Salt lowers the water's freezing point via freezing point depression. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. When you mix salt onto that layer, it slowly lowers its melting point. The more surface area salt can cover, the better the chances for melting ice. This phenomenon is called freezing point depression.
Road salt less effective when temps drop well below freezing
Salt Melt Ice Temperature The salt has to dissolve into its ions in order to work. At this temperature, your icy road generally has a thin layer of. Salt only helps if there is a little bit of liquid water available. Salt lowers the water's freezing point via freezing point depression. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. This phenomenon is called freezing point depression. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. When you mix salt onto that layer, it slowly lowers its melting point. The more surface area salt can cover, the better the chances for melting ice. The salt has to dissolve into its ions in order to work. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work.
From meltsnow.com
Salt vs. Temperature Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a little bit of liquid water available. When you mix salt onto that layer, it slowly lowers its melting point. At this temperature, your icy road generally has a thin layer of. Salt lowers the water's freezing point via freezing. Salt Melt Ice Temperature.
From lessonfullbeddington.z21.web.core.windows.net
Facts About Salt Melting Ice Salt Melt Ice Temperature When you mix salt onto that layer, it slowly lowers its melting point. The salt has to dissolve into its ions in order to work. The more surface area salt can cover, the better the chances for melting ice. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. When. Salt Melt Ice Temperature.
From dengarden.com
The Difference Between Rock Salt and Ice Melt Dengarden Salt Melt Ice Temperature At this temperature, your icy road generally has a thin layer of. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The salt has to dissolve into its ions in order to work. At 0 °c, ice melts and freezes at the same rate, so you don't see ice. Salt Melt Ice Temperature.
From sciencenotes.org
Why Salt Makes Ice Colder How Cold Ice Gets Salt Melt Ice Temperature Salt only helps if there is a little bit of liquid water available. The more surface area salt can cover, the better the chances for melting ice. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. When you mix salt onto that layer, it slowly lowers its melting point.. Salt Melt Ice Temperature.
From lessonliblandaulets.z21.web.core.windows.net
Why Does Salt Melt Ice Chemistry Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a little bit of liquid water available. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. The key is, there has to be at least a tiny bit of. Salt Melt Ice Temperature.
From jpl.nasa.gov
Educator Guide Melting Ice Experiment NASA/JPL Edu Salt Melt Ice Temperature Salt only helps if there is a little bit of liquid water available. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. Salt lowers the water's freezing point via freezing point depression. The key is, there has to be at least a tiny bit of water on the road. Salt Melt Ice Temperature.
From www.youtube.com
Salt, Sugar or Hot Water Which melts ice faster? YouTube Salt Melt Ice Temperature When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The more surface area salt can cover, the better the chances for melting ice. When you mix salt onto that layer, it slowly lowers its melting point. At 0 °c, ice melts and freezes at the same rate, so you. Salt Melt Ice Temperature.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) Salt Melt Ice Temperature At this temperature, your icy road generally has a thin layer of. This phenomenon is called freezing point depression. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Salt lowers the water's freezing point via freezing point depression. When you mix salt onto that layer, it slowly lowers its. Salt Melt Ice Temperature.
From www.themunicipal.com
Minimizing the impact of ice The Municipal Salt Melt Ice Temperature This phenomenon is called freezing point depression. Salt lowers the water's freezing point via freezing point depression. At this temperature, your icy road generally has a thin layer of. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. When you mix salt onto that layer, it slowly lowers its. Salt Melt Ice Temperature.
From sealevel.nasa.gov
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass Salt Melt Ice Temperature The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Salt lowers the water's freezing point via freezing point depression. At this temperature, your icy road generally. Salt Melt Ice Temperature.
From safepaw.com
What temperature does salt and ice melt work? Safe Paw Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. At this temperature, your icy road generally has a thin layer of. When you mix salt onto that layer, it slowly lowers its melting point. The salt has to dissolve into its ions in order to work. Salt only helps if there is a little bit. Salt Melt Ice Temperature.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer Salt Melt Ice Temperature When you mix salt onto that layer, it slowly lowers its melting point. At this temperature, your icy road generally has a thin layer of. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. The more surface area salt can cover, the better the chances for melting ice. The. Salt Melt Ice Temperature.
From doesitsnowin.com
Why Does Salt Melt Ice? Everything You Need To Know Salt Melt Ice Temperature Salt lowers the water's freezing point via freezing point depression. At this temperature, your icy road generally has a thin layer of. When you mix salt onto that layer, it slowly lowers its melting point. The more surface area salt can cover, the better the chances for melting ice. The salt has to dissolve into its ions in order to. Salt Melt Ice Temperature.
From huntingwaterfalls.com
Why Does Salt Melt Ice Faster Than Sugar? SCIENCE EXPLAINED Salt Melt Ice Temperature Salt only helps if there is a little bit of liquid water available. Salt lowers the water's freezing point via freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. At 0 °c, ice. Salt Melt Ice Temperature.
From snowicesalt.com
Ice Melt Comparison Chart Snow & Ice Salt & Chemicals Unlimited, LLC Salt Melt Ice Temperature When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. Salt lowers the water's freezing point via freezing point depression. The salt has to dissolve into its ions in order to work. Salt only helps if there is a little bit of liquid water available. At this temperature, your icy. Salt Melt Ice Temperature.
From www.slideserve.com
PPT Why Do We Add Salt to Ice? PowerPoint Presentation, free download Salt Melt Ice Temperature Salt only helps if there is a little bit of liquid water available. The salt has to dissolve into its ions in order to work. This phenomenon is called freezing point depression. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. When you mix salt onto that layer, it. Salt Melt Ice Temperature.
From huntingwaterfalls.com
Why Does Salt Make Ice Colder? THE ACTUAL REASON Salt Melt Ice Temperature When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The salt has to dissolve into its ions in order to work. At this temperature, your icy road generally has a thin layer of. When you mix salt onto that layer, it slowly lowers its melting point. The more surface. Salt Melt Ice Temperature.
From www.worldatlas.com
Why Does Salt Melt Ice? WorldAtlas Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. This phenomenon is called freezing point depression. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from. Salt Melt Ice Temperature.
From safepaw.com
What temperature does salt and ice melt work? Safe Paw Salt Melt Ice Temperature When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. At this temperature, your icy road generally has a thin layer of. When you mix salt onto. Salt Melt Ice Temperature.
From www.10tv.com
How effective is salt at melting ice as temperatures fall? Salt Melt Ice Temperature At this temperature, your icy road generally has a thin layer of. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. The salt has to dissolve into its ions in order to work. This phenomenon is called freezing point depression. When you mix salt onto that. Salt Melt Ice Temperature.
From www.northeastnursery.com
Is All Ice Melt Created Equal? Northeast Nursery Salt Melt Ice Temperature At this temperature, your icy road generally has a thin layer of. Salt only helps if there is a little bit of liquid water available. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. This phenomenon is called freezing point depression. The salt has to dissolve. Salt Melt Ice Temperature.
From safepaw.com
What Temperature Does Salt Melt Ice? Science Behind Salt and Ice Salt Melt Ice Temperature This phenomenon is called freezing point depression. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. The more surface area salt can cover, the better the chances for melting ice. Salt lowers the water's freezing point via freezing point depression. When you mix salt onto that. Salt Melt Ice Temperature.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) Salt Melt Ice Temperature The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Salt lowers the water's freezing point via freezing point depression. The salt has to dissolve into its ions in order to work. This phenomenon is called freezing point depression. At 0 °c, ice melts and freezes at. Salt Melt Ice Temperature.
From northrockmineral.com
Ice Melt vs. Rock Salt Snow Joe, LLC. Salt Melt Ice Temperature Salt only helps if there is a little bit of liquid water available. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. The salt has to. Salt Melt Ice Temperature.
From aquahow.com
The Unexpected Trick for Melting Ice Water Softener Salt AquaHow Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression. At this temperature, your icy road generally has a thin layer of. At 0 °c, ice melts and freezes at the same rate, so you don't. Salt Melt Ice Temperature.
From www.farmersalmanac.com
How to Melt Ice Naturally Farmers' Almanac Plan Your Day. Grow Your Salt Melt Ice Temperature This phenomenon is called freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. At this temperature, your icy road generally has a thin layer of. The more surface area salt can cover, the better the chances for melting ice. The salt has to dissolve into its ions in order to work. At 0. Salt Melt Ice Temperature.
From midwestsalt.com
ChartLiquidIceMelt Midwest Salt Salt Melt Ice Temperature Salt only helps if there is a little bit of liquid water available. The more surface area salt can cover, the better the chances for melting ice. When you mix salt onto that layer, it slowly lowers its melting point. The key is, there has to be at least a tiny bit of water on the road for freezing point. Salt Melt Ice Temperature.
From www.newscentermaine.com
Road salt less effective when temps drop well below freezing Salt Melt Ice Temperature At this temperature, your icy road generally has a thin layer of. The salt has to dissolve into its ions in order to work. When you mix salt onto that layer, it slowly lowers its melting point. This phenomenon is called freezing point depression. The more surface area salt can cover, the better the chances for melting ice. When rock. Salt Melt Ice Temperature.
From www.sciencekiddo.com
Why Salt Melts Ice Easy Science for Kids Science Kiddo Salt Melt Ice Temperature When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. When you mix salt onto that layer, it slowly lowers its melting point. Salt only helps if there is a little bit of liquid water available. At this temperature, your icy road generally has a thin layer of. This phenomenon. Salt Melt Ice Temperature.
From learningnadeausurfier.z21.web.core.windows.net
How Do Salt Melt Ice Salt Melt Ice Temperature Salt lowers the water's freezing point via freezing point depression. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. The more surface area salt can cover, the better the chances for melting ice. At this temperature, your icy road generally has a thin layer of. The key is, there. Salt Melt Ice Temperature.
From lessonberginmoabites.z21.web.core.windows.net
How Does Salt Interact With Ice Salt Melt Ice Temperature Salt only helps if there is a little bit of liquid water available. When you mix salt onto that layer, it slowly lowers its melting point. The salt has to dissolve into its ions in order to work. Salt lowers the water's freezing point via freezing point depression. At 0 °c, ice melts and freezes at the same rate, so. Salt Melt Ice Temperature.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. The salt has to dissolve into its ions in order to work. When rock salt is mixed in the water, it lowers the. Salt Melt Ice Temperature.
From www.branchor.com
Why Does Salt Melt Ice? Understanding the Science and Benefits of Using Salt Melt Ice Temperature At this temperature, your icy road generally has a thin layer of. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression. When rock salt is mixed. Salt Melt Ice Temperature.
From ninjadeicer.com
Rock Salt vs Ice Melt What’s the Difference? Ninja DeIcer Salt Melt Ice Temperature This phenomenon is called freezing point depression. At this temperature, your icy road generally has a thin layer of. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it. Salt Melt Ice Temperature.
From huntingwaterfalls.com
Why Does Salt Make Ice Colder? THE ACTUAL REASON Salt Melt Ice Temperature When you mix salt onto that layer, it slowly lowers its melting point. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. At 0 °c, ice. Salt Melt Ice Temperature.