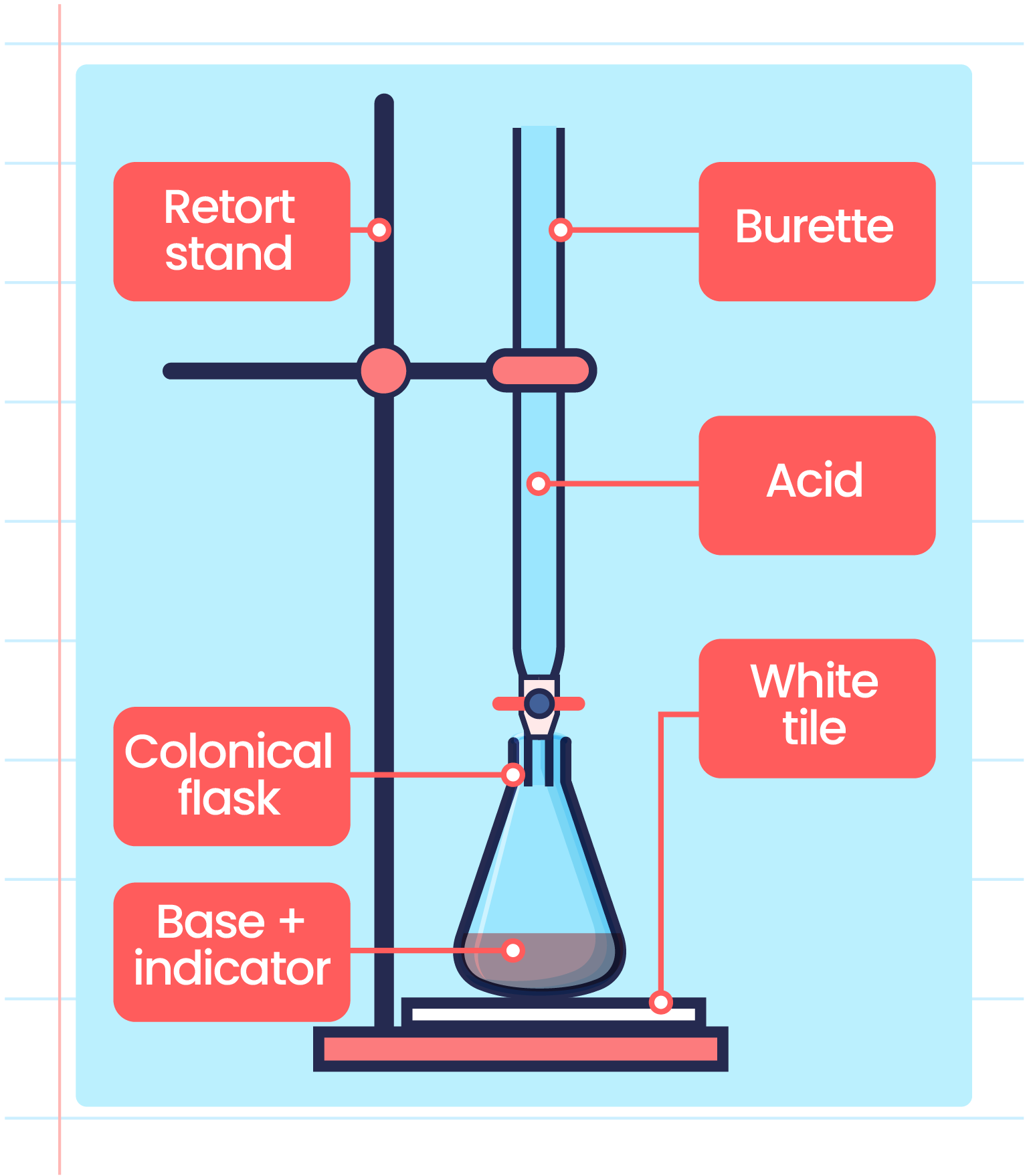
Titration Kake Bole . A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
from ar.inspiredpencil.com
এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. There are two basic types of acid base titrations, indicator and potentiometric.
Titration Setup Diagram
Titration Kake Bole A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
From www.youtube.com
Back titration YouTube Titration Kake Bole In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution.. Titration Kake Bole.
From fyovrtuly.blob.core.windows.net
Computer Hardware Kake Bole at James Romero blog Titration Kake Bole A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. There. Titration Kake Bole.
From www.dreamstime.com
Redox Titration with Burette and Pipette Stock Vector Illustration of Titration Kake Bole A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In an indicator based titration you add another chemical. Titration Kake Bole.
From www.youtube.com
কোণ কাকে বলে Kon Kake Bole উন্নতি কোণ কাকে বলে অবনতি কোণ কাকে বলে Titration Kake Bole A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. A titration is a laboratory technique used to precisely measure molar concentration of. Titration Kake Bole.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Kake Bole A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. There are two basic types of acid base. Titration Kake Bole.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Kake Bole A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. There are two basic types of acid base titrations, indicator and potentiometric. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a. Titration Kake Bole.
From www.studocu.com
Basics of titrations AGC Book 20 Cyan Basics of Titration Titration Titration Kake Bole এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique. Titration Kake Bole.
From www.youtube.com
বিপ্রতীপ কোণ কাকে বলে উদাহরণ ও চিত্রসহ Biprotip Kon Kake Bole ও এর Titration Kake Bole A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. এখানে টাইট্রেশন কাকে. Titration Kake Bole.
From boighar.in
Kake Bole Betar Natya Boighar Dot In Titration Kake Bole A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. A titration is. Titration Kake Bole.
From www.youtube.com
প্রক্রিয়া প্রতীক কয়টি ও কি কি/যোগ,বিয়োগ,গুন,ভাগ কাকে বলে/jog kake Titration Kake Bole A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a volumetric technique. Titration Kake Bole.
From www.youtube.com
মৌলিক সংখ্যা কাকে বলে মৌলিক সংখ্যা কি Moulik Sonkha Kake Bole YouTube Titration Kake Bole এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a. Titration Kake Bole.
From www.exoticindiaart.com
Yoga Kake Bole (Bengali) Exotic India Art Titration Kake Bole In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is. Titration Kake Bole.
From beingchemist.com
Applications and Importance of Acid Base Titration Being Chemist Titration Kake Bole A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A. Titration Kake Bole.
From www.pw.live
Titration Curve Of Amino Acids Titration Kake Bole There are two basic types of acid base titrations, indicator and potentiometric. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a. Titration Kake Bole.
From www.vrogue.co
Standardization And Acid Base Titration Lab Part 1 Ca vrogue.co Titration Kake Bole In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. There are two basic types of acid base titrations, indicator and potentiometric. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a technique used in chemistry to. Titration Kake Bole.
From app.jove.com
Complexometric Titration Overview Analytical Chemistry JoVe Titration Kake Bole In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution.. Titration Kake Bole.
From fi.pinterest.com
Quantitative Chemistry a) Calculating Formulas Mass b) Calculating Titration Kake Bole A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. There are two basic types of acid base titrations,. Titration Kake Bole.
From www.youtube.com
সমকোণ কাকে বলে চিত্র ও উদাহরণসহ Somokon Kake Bole ও এর বৈশিষ্ট্য Titration Kake Bole A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. Titration Kake Bole.
From www.vrogue.co
Wie Funktioniert Titration vrogue.co Titration Kake Bole A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. Titration Kake Bole.
From www.alamy.com
Titration pipette hires stock photography and images Alamy Titration Kake Bole এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration is a volumetric technique. Titration Kake Bole.
From www.youtube.com
কোণ কাকে বলে Kon Kake Bole কোণ কোণ কাকে বলে সংজ্ঞা Kaun Kake Titration Kake Bole এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. Titration Kake Bole.
From www.pinterest.com.au
titration process and its application Chemistry practical, Teaching Titration Kake Bole A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte). Titration Kake Bole.
From www.tes.com
KS3 GCSE Chemistry Introduction To Titrations Lesson Presentation and Titration Kake Bole In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is. Titration Kake Bole.
From thenoveldifference.com
Titration and Neutralization 6 Fancy Difference Titration Kake Bole A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. A titration is a volumetric technique in which a solution of one reactant (the. Titration Kake Bole.
From www.vrogue.co
A Comparison Of Original Titration Curve And Calculat vrogue.co Titration Kake Bole There are two basic types of acid base titrations, indicator and potentiometric. এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration is a laboratory technique used to precisely measure molar concentration of an. Titration Kake Bole.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Kake Bole There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a. Titration Kake Bole.
From www.youtube.com
Titrations Analytical Techniques Me Study YouTube Titration Kake Bole এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are two basic types of. Titration Kake Bole.
From www.vrogue.co
Titration Principle Working And Application vrogue.co Titration Kake Bole There are two basic types of acid base titrations, indicator and potentiometric. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed. Titration Kake Bole.
From www.youtube.com
Britto kake bole বৃত্ত কাকে বলে ও বৃত্তের খুঁটিনাটি YouTube Titration Kake Bole A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence. Titration Kake Bole.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Titration Kake Bole এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. In an indicator based titration you. Titration Kake Bole.
From www.youtube.com
Potentiometric titration (विभवमितीय अनुमापन), acid base, redox Titration Kake Bole A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. In an indicator based titration you add another. Titration Kake Bole.
From mungfali.com
Polyprotic Acid Titration Curve Titration Kake Bole There are two basic types of acid base titrations, indicator and potentiometric. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed. Titration Kake Bole.
From resource.studiaacademy.com
Acids, Alkalis and Titrations Studia Academy Resources Titration Kake Bole এখানে টাইট্রেশন কাকে বলে বা টাইট্রেশন সংজ্ঞা, টাইট্রেশন এর সূত্র এবং টাইট্রেশন পদ্ধতি. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. There are two basic types of. Titration Kake Bole.
From www.slideshare.net
Tang 07 titrations 2 PPT Titration Kake Bole A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. There are two. Titration Kake Bole.
From www.youtube.com
পূরক কোণ কাকে বলে উদাহরণ ও চিত্রসহ Purok Kon Kake Bole ও এই কোণের Titration Kake Bole There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration. Titration Kake Bole.